Developing a diagnostic test is one thing. Bringing it to the market while taking into account the product’s value for different stakeholders is a very different task. While clinicians might estimate the value of a test by its impact on the patient, many more factors influence the success of a diagnostic product. All stakeholders, from caregivers to patients to manufacturers to payers and policy makers, expect different benefits from a product, and only if the diagnostic tool meets the criteria of all these groups will it thrive in the market.
Although industry insiders will be able to value the merits of a certain diagnostic test, this is often unclear for external parties judging the product. By performing a health economic analysis, a product’s clinical and economical value can be clearly presented, allowing companies to inform and convince stakeholders of the benefits of a diagnostic tool. Convincing doctors, patients and payers of these benefits is usually harder for a diagnostic product than for a therapeutic product.
Diagnostics are notoriously undervalued. They take up a mere 2% of the healthcare budget while 70% of healthcare decisions are influenced by the outcome of diagnostic tests!
On top of that, insurance companies and governments who pay the healthcare bills fear that more diagnostic tools will lead to higher use of therapeutics. This is an important misconception, since diagnostics allow a more efficient use of the healthcare budget. Tests that succeed in identifying the right therapy for the right patient or in detecting diseases early on likely improve patient outcomes and may drastically reduce costs. Diagnostics aim to remove the trial and error workflow or ‘casino approach’ of treatment options.”
Panaxea is a consultancy firm for health care innovators such as pharmaceutical and biotech companies, and it specializes in health economic analyses. Many companies lack market access insight, and Panaxea helps these enterprises to define the true value of their products. Panaxea aids companies in making well-informed decisions and advises them on how to bring products to the market in a quick and efficient way. Their health economic models can reveal the clinical and societal impact of innovations and the specific needs of the market, allowing them to guide the R&D process to deliver a product suited to those needs.
“It often occurs that stakeholders aren’t convinced of a diagnostic product. Our analyses articulate the added value, both in clinical and economical, long and short terms. This supports acceptance and adoption of the products by the caregivers, policymakers and the market. While these health economic analyses are already mandatory for therapeutics, no clear regulations are in place for diagnostic products. This will most likely change soon, since the value of these assessments is gradually being recognized by all involved parties. Budget cuts in healthcare raise the need for more informed and rational decisions to ensure that only innovations with a significant impact on the healthcare system are allowed. A health economics study is essential to guide these decisions. Also, manufacturers can benefit from an assessment. If we do these studies in an early stage of development, it can also offer recommendations for adjusted research activities, to tailor a product to the needs of all stakeholders,” says Isabelle Lepage-Nefkens, CEO of Panaxea.
These needs and the value of innovations most surely differs between stakeholders. Caregivers and doctors require an innovation to simplify the workflow and need scientific evidence of higher accuracy or other advantages the test may offer. Payers and policy makers are more concerned with the pricing and budget impact of these innovations and the associated societal impact they can have, since this group of stakeholders is responsible for the rational use of healthcare resources.
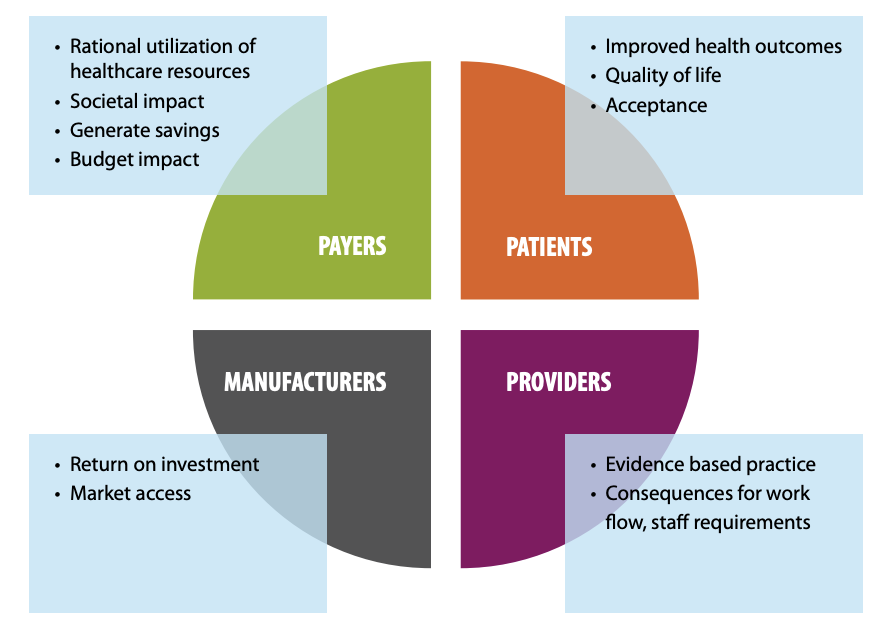 The evaluation of the clinical and economic value of a diagnostic can be quite complex because it requires detailed knowledge of existing and emerging steps in the care pathway, starting from diagnosis all the way to therapy. Although a single stakeholder might experience short-term negative effects (for example, budget impact on medical lab or payers), the final health outcomes and societal benefits typically occur in the longer term. The different timings of these outcomes means that stakeholders do not experience the same benefits or burdens, and as such they have different decision drivers and evidence needs.
The evaluation of the clinical and economic value of a diagnostic can be quite complex because it requires detailed knowledge of existing and emerging steps in the care pathway, starting from diagnosis all the way to therapy. Although a single stakeholder might experience short-term negative effects (for example, budget impact on medical lab or payers), the final health outcomes and societal benefits typically occur in the longer term. The different timings of these outcomes means that stakeholders do not experience the same benefits or burdens, and as such they have different decision drivers and evidence needs.
Also, patients are gaining in importance, and their view on value is critical. A patient’s opinion can determine whether a test is accepted. He or she might not be willing to endure a painful process to get a diagnosis of a severe disease due to anxiety. In this context, patient outcomes, quality of life and comfort are additional factors that need to be considered. Lastly, the product manufacturer needs its product to find market access and ensure a return on investment.
This all demonstrates how multiple criteria, exceeding clinical relevance, need to be fulfilled by a diagnostic assay. Panaxea is dedicated to mapping these possible challenges and presenting possible solutions to optimize a diagnostic product for all stakeholders. Additionally, point-of-care (POC) testing, telemonitoring and ICT-linked diagnostics are causing a revolution in healthcare and diagnostics. In this context, Panaxea is well situated to anticipate these innovations and has already built expertise in the conceptualization, evaluation and implementation of these entirely new models of care delivery.
This article appears in the BioVox White Paper on In Vitro Diagnostics, May 2016. Download the complete work here.


