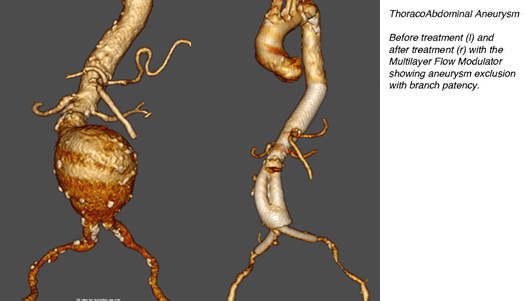
The continuous flow in jet-induced fountains inspired Nourredine Frid, CEO at Cardiatis (Isnes), to apply the theory of fluid mechanics to blood vessels. By converting turbulence to laminar flow in a blood vessel, an aneurysm (a localized dilation of a vessel wall) could be treated. In other words, by regulating the speed of the blood flow, the negative effect of the stress induced by the turbulence on the arterial wall could be reduced. Based on this principle, Cardiatis, in cooperation with Walbiostent, developed a novel type of stent called the Multilayer Flow Modulator to treat complex aneurysms; the MFM® can be inserted through a minimally invasive procedure. They are the first in their field!
Each year 120,000 endovascular aneurysm repair procedures are performed worldwide. The main purpose of endovascular aneurysm repair is to prevent death from a ruptured aneurysm, whether cerebral, peripheral or aortic. Ideally, this is done by completely removing the aneurysm while protecting the circulation in branches and collateral vessels to avoid stroke, spine and visceral ischemia.
Having worked with companies active in the medical treatment of cardiovascular diseases since the 1990s, Frid had the medical know-how — and the innovative mind — to consider fluid mechanics as a potential and global solution for aneurysms, as well as for aortic dissection (a similar disease). Thanks to the four-year, BioWin-supported Walbiostent* project, Frid was able to design and develop an advanced new generation of multilayer flow modulator stents. Manufacturers from Lyon — together with a consortium** of research centers and engineers from Belgium — developed an apparatus resembling a highly intricate mesh-like structure that consists of thousands of intertwined gears.
A self-expanding stent
Composed of three layers of braided wire (Phynox), the Cardiatis MFM® regulates the blood flow inside the aneurysm. Its three-dimensional structure simultaneously regulates hemodynamic flow within the affected artery, reduces local stress within the aneurysm and laminates the blood flow. As a result, a thrombus is formed and the aneurysm is able to close physiologically. “Moreover,” Frid adds, “placement of this stent only requires a short, minimally invasive procedure that can be done under local anesthesia. This means there is no trauma and the patient can return home the next day.”
What makes it different from traditional stents?
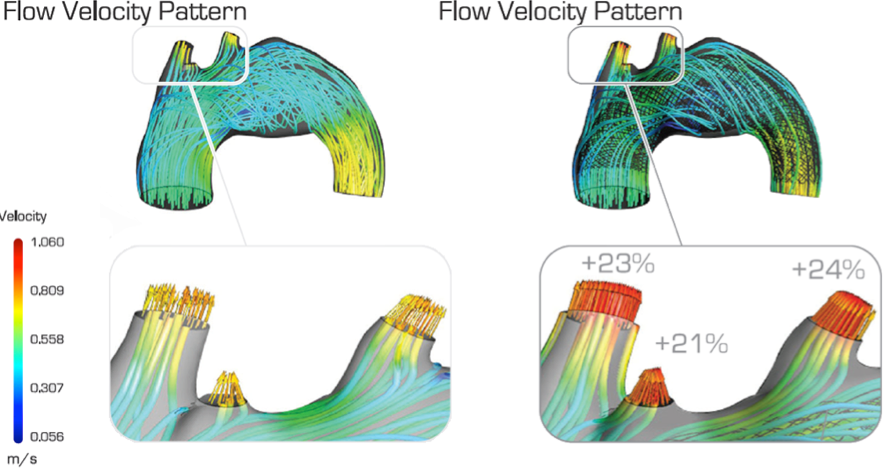
Conventional stents (commonly named stent-grafts) are considered the standard form of care in the industry. Made from an impermeable polyester fabric, they isolate aneurysms from blood flow. However, due to their rigid structure, these devices can create massive complications in veins or arteries close to the deployment zone, such as paraplegia, renal malfunction, failure of visceral organs and even death. “The Cardiatis MFM® differs from these, as it naturally preserves the blood circulation in branches and collateral vessels,” Frid explains. “Test results have shown that the hemodynamic changes induced by the Cardiatis MFM® lead to reductions of over 90% in blood flow velocity within aneurysms. This is two times more than what is possible with any commercially available single layer stent.”
“Cardiatis is the only company developing a device for the treatment of all types of aneurysms (saccular, fusiform, branched and bifurcated) in all locations (cerebral, peripheral, aortic, abdominal); it is the only device promoting aneurysm healing through endothelialization while keeping the patency of branches and collateral vessels. This constitutes a paradigm shift in aneurysm treatment,” Frid says.
“I understand that some people are hesitant regarding this paradigm shift,” Frid continues. “However, this innovative technology is sparking the interest of doctors worldwide, and many scholars and key opinion leaders in the field are quickly recognizing the potential of the MFM®. At the same time, the limitations of the current stent-graft technology for the treatment of complex cases such as thoracoabdominal aortic aneurysms, aortic dissection, juxta- and pararenal aneurysms or cerebral aneurysms are becoming apparent.”
Engineering simulation to rule out all risks
“Cardiatis has now identified a reliable solution for addressing people who doubt: engineering simulation,” Frid says. “Simulation has been an indispensable design tool for the product development team at Cardiatis. It is unfeasible to perform early-stage product testing on human patients. Simulation has been a foundational solution in modeling the MFM®’s performance inside a virtual human body.” Cardiatis makes use of 4,000 two-dimensional CT scans, which are transformed into three-dimensional geometries. The engineers are currently simulating the performance of the MFM® inside a range of simulated human bodies to ensure that it will deliver consistent results for all kinds of patients.
The Cardiatis MFM® is already being used in Europe , the Middle East, South America and Asia to treat high-risk patients who are unable to benefit from traditional treatment. The engineering team at Cardiatis generates simulations that are based on the actual patients’ scans and images. Based on this information, they advise the medical team on the procedures. MFM® simulations have proven remarkably accurate.
Ready to conquer the world
The traditional endovascular aneurysm repair market now faces a lower growth rate — around 5% per annum, as opposed to double-digit growth in the early 2000s — due to the complications that arise alongside this treatment. “A strong momentum is building up for Cardiatis’ MFM® platform, which is the sole technology that offers viable options for patients with complex pathologies,” Frid says. The market potential for the treatment of complex aneurysms is estimated to be at around 1.5 billion dollars per year, excluding cerebral aneurysms and aortic dissection, which may fuel additional growth. “With a total of nearly 4,600 implant procedures performed since 2006 with clinically proven benefits to the patients, Cardiatis aims to become a leader in the industry,” Frid concludes.
The company is currently initiating clinical studies and registration procedures in Europe and the rest of the world to further strengthen its evidence base, including in Belgium, where its products are not yet reimbursed but are offered for studies.
“Thanks to fluid mechanics, this revolutionary product could indeed prove to be the unique and most effective solution to overcoming what for years has been known as the silent killer of so many.”
*The Walbiostent project had been focusing its study on the interaction between stent, arterial wall and blood, while Cardiatis’ involvement in the project has been focused on the effect of the multilayer stent relative to shear stress flow that prevents hyperplasia and anti-platelet aggregation.
**Consortium comprises companies (Cardiatis [Coordinator]), BIO.be and research centers: FUNDP (URBC), UCL (ILS), CHU Charleroi, ULB (Laboratory of Experimental Medicine), Ecole Polytechnique (Faculty of Engineering, Mons).
References
Ansys, Dimensions, Volume I, Issue 1, 2016: ‘Innovative at heart’