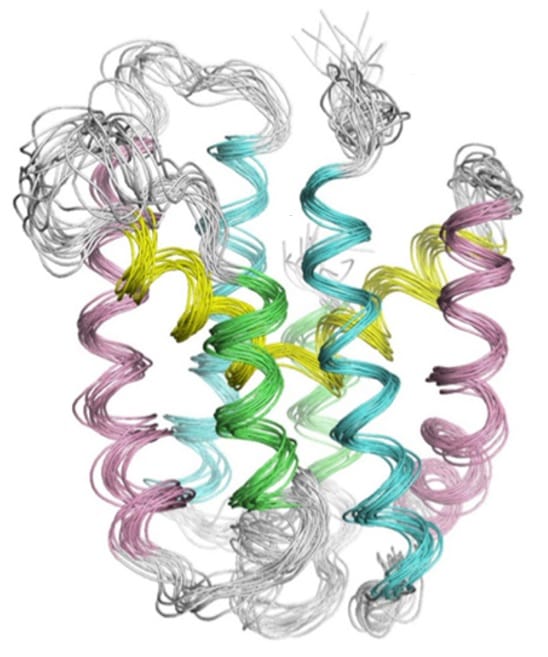
Transmembrane reductases form an important group of enzymes in the thiol-redox chain, the mechanism by which bacteria cope with oxidative stress. Despite the significance of these electron carriers, their detailed structure had never been described. Recently, investigators at the UCL and the de Duve institute, in collaboration with groups at Harvard Medical School, elucidated the structure of the archaean CcdA. Their impressive technical achievement was published in Nature structural and molecular biology.
While the bacterial periplasm is an oxidizing environment, the cytoplasm is reducing. The transfer of electrons across the inner membrane is essential for energy conservation and defense against oxidative stress. This task is taken up by the transmembrane reductases, which bind electron-carrying proteins at both sides of the membrane and transfer reducing power via a single cystein pair. Yet, the exact manner in which these reductases relayed electrons was unknown.
In order to solve this mystery, the researchers chose to determine the structure of the representative transmembrane reductase CcdA. While CcdA originates from the archaeon Archaeoglobus fulgidus, it is highly homologous to other bacterial transmembrane reductases and thus is thought to have a similar mechanism for transporting electrons. Determining the structure of membrane proteins is a burdensome task, regardless of how simple or complex the protein may be. CcdA is a relatively simple protein, but uncovering its structure still proved to be a technical challenge that took 5 years to complete.
The four-step model
The NMR structure of the protein enabled the researchers to propose a four-state mechanism of action. According to this model, electrons are passed from the reduced thioredoxin A in the cytoplasm to the cystein residues present on the transmembrane CcdA and finally to the periplasmic thioredoxin A protein. During the passage of electrons, CcdA undergoes several conformational changes to present one of the two cysteine residues to either side of the membrane. The way in which CcdA coordinates substrate binding and electron relay is unique among electron transporting proteins, making this a remarkable discovery.
Reference
Williamson, Jessica A., et al. “Structure and multistate function of the transmembrane electron transporter CcdA.” Nature structural & molecular biology (2015).