Stem cells for heart failure
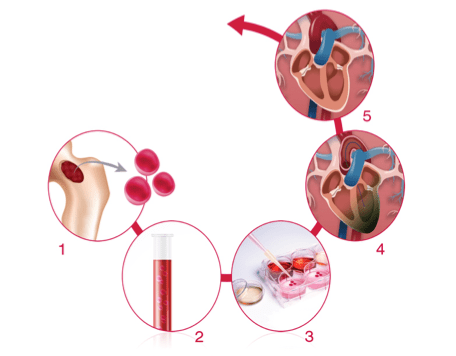
Multiple years of research collaboration between the Mayo Clinic (Minnesota, USA), Celyad (previously known as Cardio3 BioSciences) and the Cardiovascular Centre in Aalst led to the development of a Cardiopoietic platform. This is a combination of signaling proteins that are involved in the transformation of generic adult stem cells, e.g. human mesenchymal stem cells (hMSC) into cardiac progenitor cells. First, the bone marrow is drawn from the patient and then the stem cells are isolated and multiplied until their number reaches the desired number. Subsequently, they are treated with the Cardiopoietic combination of cytokines and growth factors in order to differentiate into cardiac progenitor cells cells. The Cardiopoiesis platform mimics the process of cardiac development in the embryo, without the need to modify the genome of the cell. After this ex vivo transformation, the cells are infused into the patient’s heart where they become functional cardiomyocytes and endothelial cells. These newly differentiated cells can help to repair the damaged heart disease, such as for example in ischemic heart disease – where the lack of blood flow starves the heart cells of oxygen and nutrients. The C-Cure® process takes approximately 40 days after bone marrow extraction to be ready for injection in the patient’s heart. It takes about 28 days between selection of the stem cells and realization of the end product.
Time to approval
Patient enrollment for CHART-1 was completed in March 2015 and the efficacy read-out is expected by the middle of 2016. If it is successful, the first marketing approval may possibly be obtained by end 2017. This is the first time that lineage-guided stem cells have been used in patients for targeted regeneration of a failing organ. “With C-Cure®, Celyad aims to become the first company with an approved regenerative product for ischemic heart failure and to provide a leading edge cell therapy to patients all across the globe.” – explains Cristian Homsy, CEO of Celyad.
Also, read about Celyads discoveries for cancer treatment.