More than meets the eye
For the few individuals who don’t know who Biocartis is, this is the company behind the Idylla™ platform. This molecular diagnostic system profiles cancer mutations or detects infectious pathogens, depending on the cartridge used, in a fast and accurate way. Whereas performing comparable tests with previously existing technology took days or weeks, Idylla™ provides a fully automated sample-to-result diagnosis in a matter of hours, with only a few minutes of hands-on time. Idylla™ features the kind of sleek, minimalist design that helped make Apple products so popular. Beneath its smooth shell, however, Idylla™ is robust, sensitive and speedy, giving a hint of what tomorrow’s healthcare technology will look like.
We see large medical centers starting studies to see if they can offer cancer patients a one-day visit to the hospital, based on the incredibly fast turnaround time of Idylla™.
Keeping promises
We had the pleasure to look back on 2015 with Biocartis’ Chief Scientific Officer, Geert Maertens. When considering the past year, the introduction of Biocartis to the stock market immediately springs to mind: “The IPO had a huge impact on the company, as it has given us the resources to play on the global field. Despite our six successful financing rounds, we were still looking to increase our range. If you want to develop a platform and expand the menu of tests and do the manufacturing, all while becoming a global player, that requires capital. The IPO has granted us those means. Biocartis is now represented in 55 countries worldwide, and we can continue releasing new assays at a considerable pace. Another benefit of a public offering is the visibility it creates, not only for sales but also in terms of attracting new investors.”
“We’re proud to have delivered on the promises we made during the IPO and even to have taken it slightly further than that. Our first liquid biopsy test, the Idylla™ ctBRAF Mutation Assay, was originally not on the program, but we still managed to add it to the menu before the end of the year. Also, the installation of Idylla™ instruments surpassed our expectations. We added 83 instruments to our installed base in 2015, exceeding our target of 75 additional instruments.”
Tracking down cancer and bugs
Now that Idylla™ is gaining momentum, it’s of paramount importance to expand the menu of tests the platform can run. While Idylla™ cartridges can be developed for any genetic test, Biocartis chose to focus on oncology and infectious disease panels, respectively the fastest growing and largest market segment within molecular diagnostics. Maertens explains:
“It’s essential to build a core menu to convince potential customers to use the system. One or two tests do not suffice. We’re currently working on 17 R&D projects to rapidly increase the number of tests that Idylla™ can run over the coming years.”
We’ve seen companies bet big on one important product and then get into trouble when the community isn’t supportive enough.
“In oncology, our first focus, developing tests for established cancer markers, lowers the threshold for the scientific community to adapt our Idylla™ platform. We didn’t want to develop entirely new biomarker panels and still need to convince the world that these panels have value. For a young company, that’s an uphill battle that easily costs €20 to €30 million. We’ve seen companies bet big on one important product and then get into trouble when the community isn’t supportive enough. We chose assays for known relevant markers, which are sure to be reimbursed. By gradually improving these assays, expanding them with other mutations and making them more sensitive and fully automated, we were able to bring something pretty unique to the market that has already attracted the attention of several pharmaceutical companies.
In the context of infectious diseases, we aim to develop syndromic panels. For example, next year we are launching our respiratory panel, and later that year a meningitis panel. Here we can leverage an important advantage of Idylla™: Its large capacity for multiplexing. We can detect a set of approximately 25 pathogens in a single sample, measuring DNA and RNA, bacteria and viruses in one assay.”
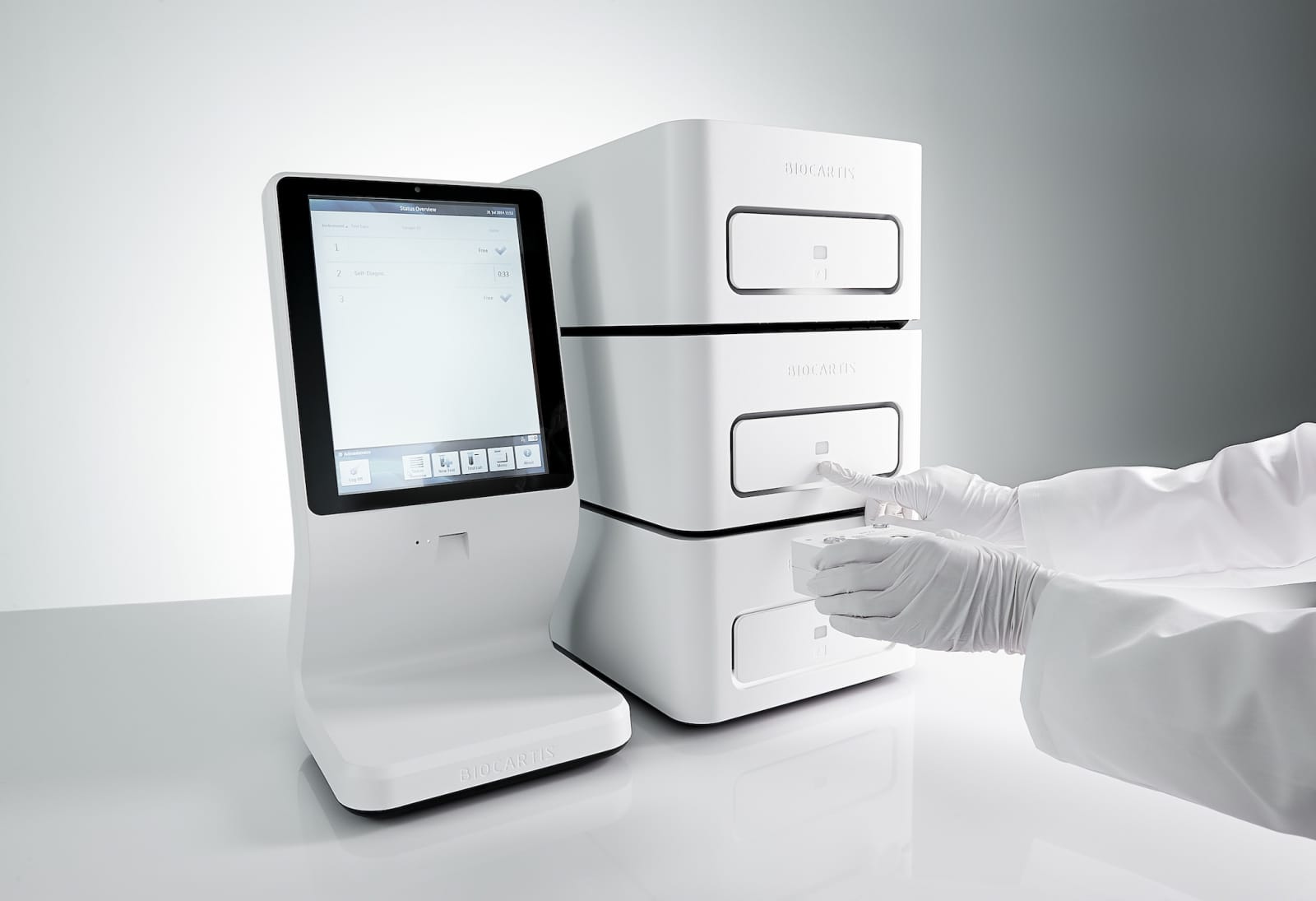
Meanwhile, Idylla™ is already changing and shaping the diagnostics landscape: “We see large medical centers starting studies to see if they can offer cancer patients a one-day visit to the hospital, based on the incredibly fast turnaround time of Idylla™. Within that single day, all necessary tests are carried out, the results are collected and hopefully by the end of the day the patient can leave the hospital with the most suitable treatment. That’s a revolution for cancer diagnostics! Today, patients with colon cancer sometimes have to wait from six weeks up to even two months for their results. In the meantime, these patients often undergo general chemotherapy, which is not always the best treatment, as there could be many side effects. That’s the dramatic situation for many patients, and we’re very proud to contribute to their improved wellbeing.”
What’s next?
Looking back at past successes is nice, but Biocartis keeps looking forward, and its future looks brighter than ever. The company plans to bring at least four new tests to the market each year. During 2016 and 2017, Biocartis will expand its menu of assays, or “diagnostic app store,” with both solid and liquid biopsy tests for lung and colon cancer, Ebola and MERS assays, a respiratory pathogen panel and a meningitis panel. Also, a second cartridge manufacturing line should be operational in 2017, greatly increasing production capacity. On top of all that, the Idylla™ platform is scheduled for FDA consideration around the end of the year; if it’s approved, Biocartis will introduce it to the US market. The CSO is optimistic about the company’s future:
I’ve had the pleasure to see this company grow from 7 employees back in 2009 to a team of around 300 people today.
“That’s already an astounding leap, and we’ll keep on growing in the coming years. We have so many opportunities with Idylla™, it’s almost frustrating that we have to select and prioritize which projects and assays to finish first. The possibilities are simply overwhelming. That’s also the reason why our partnerships are so important: Thanks to our partners in healthcare, both large companies such as Johnson & Johnson and smaller but important test development partners such as Fast-track Diagnostics, we can significantly accelerate the development of products in our pipeline. Collaborations are essential to our success and to our strategy in the future. We wish to move toward a sustainable healthcare ecosystem, with faster, more accurate diagnoses creating a positive impact for all stakeholders — including patients, care providers, payers, industry and society as a whole.”


