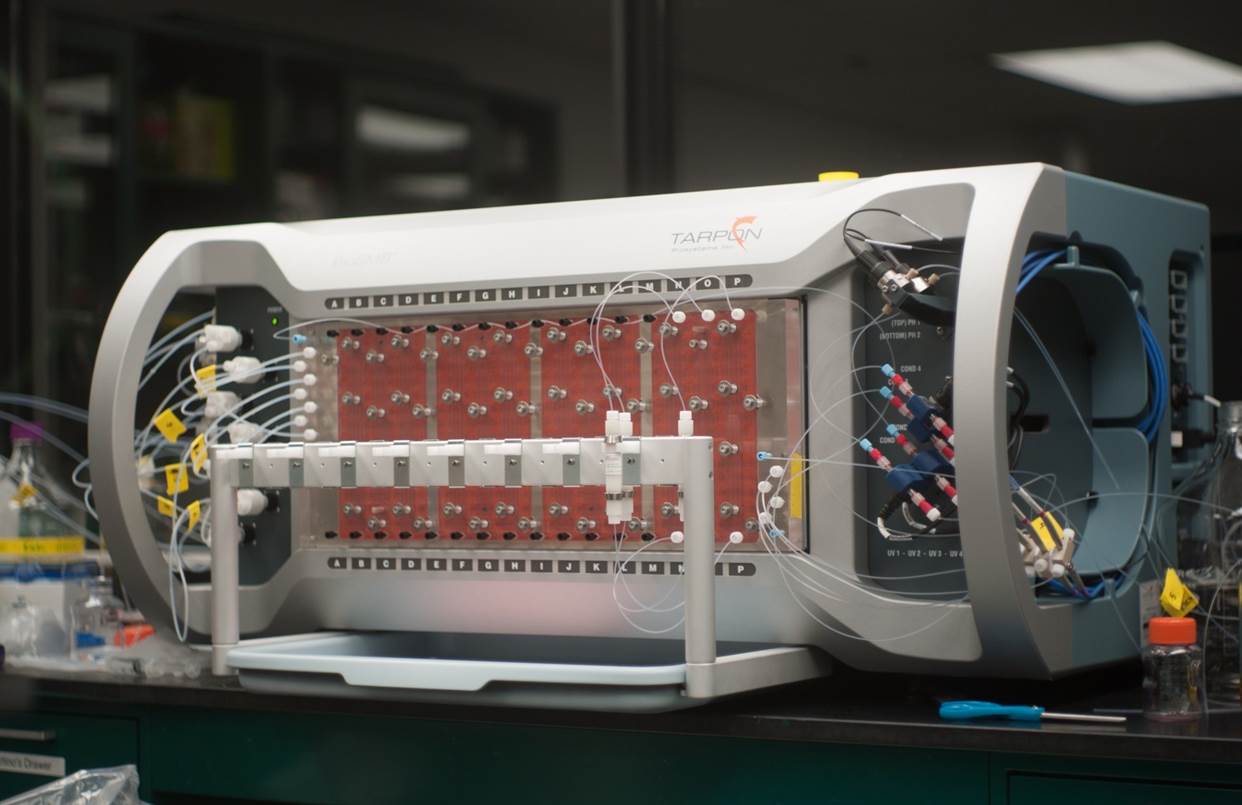
Single-use continuous chromatography
In a standard chromatography purification process, a relatively large column —sometimes up to more than 1 meter wide and a couple of meters high— is used for batch purification. This type of column is extremely expensive, and a long contact time between the resin and the product is necessary. A Dutch-US company, Tarpon Biosystems, developed the BioSMB continuous chromatography system, which Pall acquired one year ago. The BioSMB system makes use of several small chromatography columns that are controlled by a fully automated apparatus, the Cadence™ BioSMB. “Once everything is connected and programmed, the process runs automatically,” adds Tessa Johnson, technical marketing manager at Pall.
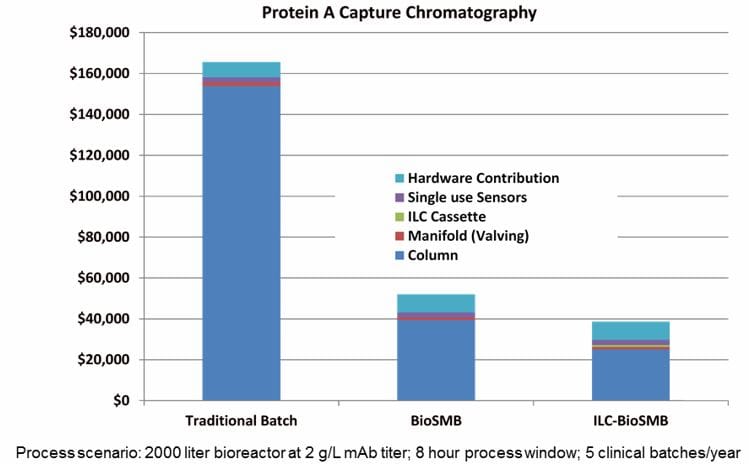
A reduction of 3 to 5 times of chromatography resin and/or sorbents can be reached, which is a huge cost saving if you consider that the purification step is 50% of the total process cost.
After production, the columns and tubes are disposed of, as these are single-use components. Single-use systems offer several economic advantages; for example, cleaning, cleaning validation, and —in this particular case— regeneration of the resin are not required, which per se is non-economical. (Read more about single-use benefits in this article on single-use bioreactors).
Huge cost savings
Compared to a batch process, the BioSMB continuous chromatography process is faster and has a higher yield. The smaller columns also require much less resin than a batch chromatography column to obtain the same or an even better product purification result, improving the overall cost efficiency. Tessa Johnson comments: “A reduction of 3 to 5 times of chromatography resin and/or sorbents can be reached, which is a huge cost saving if you consider that the purification step is 50% of the total process cost.” Also, the process is monitored precisely, which results in a higher standard of quality and a better consistency of quality, very much in line with the QbD (quality by design) requirements of the industry.
Just test it
Merck in the US (known as MSD in Europe) and Bayer have already proved that the adoption of this new technology brings major benefits for some of their monoclonal antibody production processes. “Companies who are interested can rent the Cadence™ BioSMB chromatography system for a test phase,” says Tessa Johnson. “A Pall trainer will help with the installation and manipulation of the apparatus. After a couple of weeks, the company can analyze whether this new technology is beneficial for them or not. The BioSMB is especially useful for biopharmaceutical companies who are developing new product processes, as it can be incorporated during the initial phase.”
Seminar
On April 19th, Pall will present a seminar on this topic, during which Marc Bisschops, the brain behind the BioSMB, will give a talk. Furthermore, Pall’s Cadence™ Inline Concentrator and ForteBio’s Octet System will be featured in a live demonstration. The Cadence™ Inline Concentrator is a new TFF (tangential flow filtration) module that operates in single-pass mode, allowing for a continuous concentration step. Therefore, concentration factors of 3 to 5 can be reached with this plug & play module in a simple continuous mode. Also, the many capabilities of the Octet System, which enables HCP (host cell protein) analysis in high-throughput mode, will be demonstrated. This technique allows fast and efficient analysis of this important impurity.
If you are interested in learning more about this subject, subscribe for the workshop!