It’s been known for quite some time that the energy-producing organelles in our cells, called mitochondria, carry a small amount of DNA within them. This means that a tiny fraction of our total DNA, about 0.1% to be exact, is not present in the cell nucleus, where the remaining 99.9% is stored. As is the case with nuclear DNA, faults in mitochondrial DNA (mtDNA) can give rise to a range of rare diseases.
Genetic defects that cause disease can of course be inherited by the offspring of persons carrying the defect. In this context, however, mitochondrial and nuclear DNA differ drastically. Mitochondria are only inherited from the mother, while nuclear DNA is a 50/50 combination of DNA from the mother and father. Basically, this means that all mtDNA originates from the mother, and a mother carrying a genetic defect in her mtDNA will pass this on to her offspring with 100% certainty. This causes some women to have serious fertility problems.
It takes three to tango
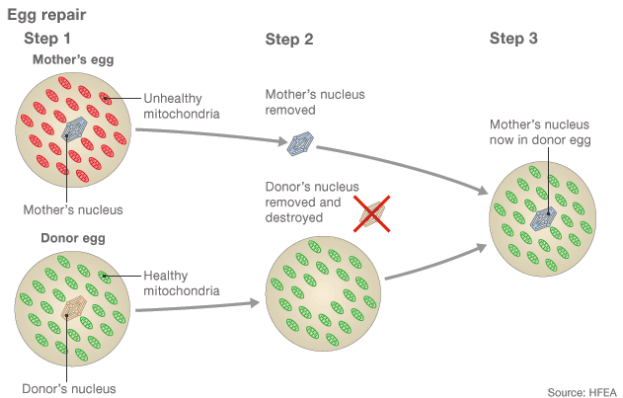
There are fertilization techniques to prevent this from happening, and these include the use of a donor egg with healthy mitochondria. The nuclear DNA of the donor egg is removed from the cell and replaced with the nuclear DNA of the mother. The result is an egg with healthy mitochondria and the mother’s nuclear DNA. Afterwards, the egg is fertilized with sperm from the father and develops into an embryo. To be clear, the DNA of the developing embryo originates from three individuals: The nuclear DNA is a mix of the mother and father’s DNA, while the 0.1% mtDNA originates from the egg donor — hence the term “three-parent babies.”
While this “spindle transfer” technique dates back to the late ‘90s, little knowledge of its outcome and long-term effects on the child is available. Only in the UK has a similar method been approved by the government, and only since February 2015. Due to the many ethical and scientific questions they pose, these methods clearly require strict regulation and further investigation.
Into this uncertain climate, the first three-parent baby was born in April 2016. The mother carried a genetic defect in her mtDNA that could cause Leigh’s disease, a severe disorder that affects the development of the nervous system. While the mother herself was healthy, she had had 4 miscarriages, and her two viable children died at 8 months and 6 years of age. Desperate to conceive a healthy child, the parents turned to Dr. John Zhang of the New Hope Fertility Center in New York.
Three’s a crowd
Because the procedures described above aren’t legal in the US, Dr. Zhang took the couple to Mexico, where regulation is absent. There, he performed the spindle transfer technique. This caused some upheaval in the scientific community, given that Dr. Zhang’s team deliberately circumvented regulations to carry out a technique with many unknown consequences.
There are multiple reasons why regulators and scientists are approaching this delicate matter cautiously. The fact that only 1 of the 5 embryos on which the method was applied developed further naturally shows that spindle transfer is a fallible technique. Scientists from all around the globe are still sorting out the issues related to this kind of technology, and there is no guarantee that the baby will be spared from health problems related to either the procedure or Leigh’s disease, the disorder the scientists were trying to avoid. While Dr. Zhang argues that “saving lives is the ethical thing to do,” world-renowned bioethicist Hank Greely counters by stating “lives are not being saved, they’re being risked.”
It’s true that the genetic content of mitochondria is minimal compared to the massive amount of DNA in the nucleus: only 17,000 base pairs of DNA encoding 37 genes in mitochondria, as opposed to more than 3 billion base pairs encoding about 20,000 genes in the nucleus. However, the two sources of genetic information appear to share an intimate bond. Experiments have shown that mitochondrial transfer also influences the expression of more than 1,000 nuclear genes, influencing a broad range of traits and indicating that there’s much more to the small portion of mtDNA than meets the eye.
As we cannot estimate today how far the consequences of mitochondrial swapping reach, the baby born in April will be watched closely as a human experiment of sorts. Interesting detail: Since the newborn is a boy, he will not pass on his “foreign” mitochondria to coming generations, and his children may not suffer the same possible issues he might. While this means the “hybrid” DNA combination will not be passed on to future generations, sadly enough there’s no certainty that the boy himself will be able to lead a healthy life. Let’s hope for the best.


