The last decade has seen the launch of multiple innovative drugs that have brought major therapeutic benefits to patients. However, these new therapies often come with a hefty price-tag: in 2012, for example, 12 out of the 13 newly approved cancer drugs were priced above $100,000 USD. These exorbitant drug prices are often caused by sky-high development costs.
According to Tufts Centre for the Study of Drug Development, the average cost of bringing a drug to the market is estimated at $2.6 billion. Most of these expenses can be attributed to the multiple clinical trials required, with each trial usually costing a company between $2 – $600 million.
Why are clinical trials so expensive?
The more patients needed, the more expensive the trial. The cost therefore varies considerably according to stage as less patients are needed earlier in the process: 5-50 patients for a phase I, 50-300 patients for phase II and 300-3000 patients for a phase III trial. The cost of enrolling a single patient has also gone up in recent years. As the number of clinical trials being conducted globally is on the rise, the competition for patients has increased sharply.
Not only the trial stage influences the number of patients needed. Clinical trial design and disease indication also contribute heavily to this factor. A novel drug for an orphan disease, where there are no other approved treatments available, will typically enroll between 10-50 patients in a single-arm, open-label trial. On the other hand, a cardiovascular or central nervous system (CNS) disease trial, where the new drug might only provide incremental benefits over other approved drugs, will enroll hundreds or even thousands of patients in a placebo or active-controlled trial to ensure statistical power.
The increasing cost of clinical trials in particular effects early stage biopharmaceutical start-ups in need of external financing to be able to conduct clinical trials for their pipeline drugs. – Rishabh Chawla, V-Bio Ventures
Due to the increase in the number of clinical trials a smaller number of patients are available, driving up the price for patient enrolment by CROs. Once the patients have been enrolled, the cost of the drug itself becomes a factor. The cost of manufacturing enough of a drug for a full clinical trial is very dependent on the type of drug in question: a small molecule inhibitor drug may only cost US$0.5 million to produce, whereas an investigational cell or gene therapy may cost millions.
Finally, the average cost of a single patient in a clinical trial differs significantly depending on the type of disease being targeted (Table 1). Certain insidious, slow progressing diseases require longer treatment and follow-up periods compared to other rapidly progressing diseases. Coupled with the use of expensive biomarkers, imaging services/monitoring tools such as computed axial tomography (CAT) and magnetic resonance imaging (MRI) required for some indications, it all adds up to an increased cost per patient.
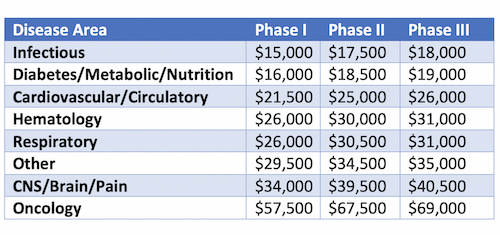
Table 1: Average cost per patient across different disease indications (Adopted from Biopharmaceutical Industry-Sponsored Clinical Trials: Impact on State Economies; 2015)
Problems for biopharmaceutical start-ups
According to Grand View Research, the cost of conducting clinical trials is rising at an annual rate of 5.7%. At this rate, clinical trials would be costing companies an extraordinary $68.9 billion by 2026. The increasing cost of clinical trials in particular effects early stage biopharmaceutical start-ups in need of external financing to be able to conduct clinical trials for their pipeline drugs.
Partly due to the increased price of clinical trials, we’re seeing larger and larger financing rounds being raised by early stage companies. These increasing financial requirements may result in high-competition among start-ups. They may also drive investors towards relatively lower risk development and clinical programs, which would be a big set-back for innovative drugs and the patients who stand to benefit from them.
Driving the prices down
There are several digital health companies that are currently attempting to use artificial intelligence (AI) to find a solution to the increasing cost of clinical trials. Flatiron Health, acquired by Roche in 2018, has developed a database of electronic and genomic records of millions of cancer patients. The database helps to optimize treatments for newly diagnosed cancer patients, propose changes to clinical trial design and identify new biomarkers.
Pharmaceutical companies, such as Pfizer, Merck and Amgen, have started using an external control arm where the control data is collected either from previous trials or from electronic health records. These innovative approaches help to reduce the number of patients per clinical trial and encourages patients to enroll in clinical trial by eliminating the risk of receiving placebo as against the experimental drug.
Our hope for the next decade is that companies not only focus on the development of novel innovative therapies, but also invest in strategic initiatives to decrease the cost of clinical trials and bring down prices. – Rishabh Chawla, V-Bio Ventures
Another possible route to decreasing trial costs may be accelerating time between clinical trial application and trial site initiation. This could be done by implementing efficient coordination between local ethics committees, trial bureaucracies and sites. The method is already being used in South Korea, where the time to complete trials has been reduced from 117 to 61 days.
Designing patient-centric trials could also help small to medium sized pharmaceutical companies increase patient enrolment. A 2019 study by the FDA revealed that over 70% of Americans are willing to participate in a clinical trial, but only about 5% of adult cancer patients are actually enrolled in a trial program. Increasing patient awareness at potential trials sites, creating better consent and education materials could help increase patient participation.
Despite their high cost, clinical trials are crucial for the development of innovative products that benefit patients. Our hope for the next decade is that companies not only focus on the development of novel innovative therapies, but also invest in strategic initiatives to decrease the cost of clinical trials and bring down prices.


