
Pieter De Wilde, Commercial Director (Medical and life-science devices / Innovation) at Unitron Group.
Innovations and the hype cycle of device development
We define an innovation as a successful realization of a creative or novel idea – that is, one that has been successfully launched on the market. The idea is the spark that drives the creation of the innovation, but it is not enough to take you to the final product, and often does not follow a straight path. There are many factors within the medical device development environment that must be considered and managed along the path towards innovation.
The Gartner Hype Cycle is a graphical and conceptual tool to assess and position the maturity and adoption level of emerging technologies and applications. The development path from innovative idea to successful innovation follows a similar trajectory with hype and disillusionment phases.
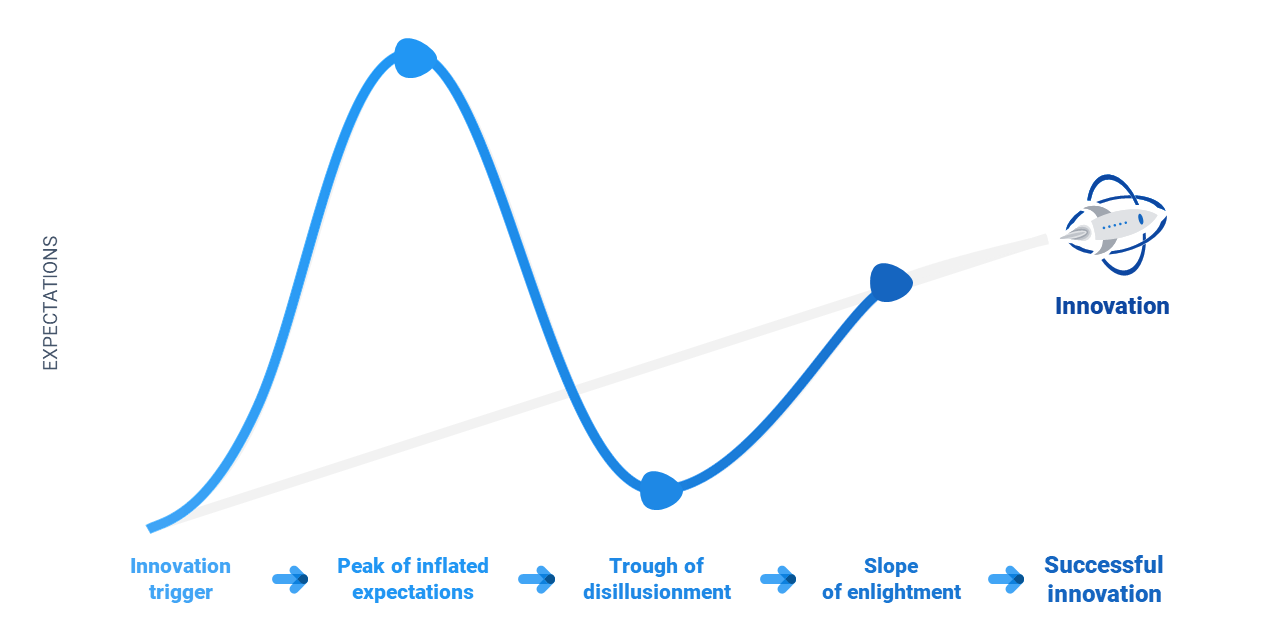
The phases of the hype cycle
Innovation trigger – This is the original idea that motivates the development of the device or technology.
Peak of Inflated expectations – The technology starts to be developed and gets some attention. People start talking about it, which creates excitement and expectations – the “hype” around the idea starts to grow. In most cases, the expectations at this stage exceed reality
Trough of disillusionment – After the peak of expectation has been reached, reality sets in and expectations decrease. Perhaps the technology doesn’t work in certain application areas, or cost-of-goods exceeds a market-worthy pricing strategy.
Slope of enlightenment – In this phase, things begin to stabilize – the technology’s benefits are clearer and become more widely understood. Technical and clinical validation is gathered and the team gears up for a successful go-to-market.
Successful innovation – The technology is brought to market. It’s now time to roll out your go-to-market plan.
This cycle may look like a challenging process to go through when developing a new product, but the good news is that it is predictable and thus manageable by making the right preparations and, most importantly, by choosing the right partners to help you navigate it.
This cycle may look like a challenging process to go through when developing a new product, but the good news is that it is predictable and thus manageable by making the right preparations and, most importantly, by choosing the right partners to help you navigate it.
A similar hype cycle can be applied to life sciences developments, read this article by V-Bio Ventures to find out more.
Managing the cycle
Probably one of the biggest risks during innovative device development is to not understand or manage the path towards implementation well. Here, we will focus our insights on three important areas: investor relations, regulatory and technology considerations.
Investor relations
Creating new technology costs money, and lots of it! It proves to be a challenging task over and over, and getting the right investors interested in your product at the right time is critical. Moreover, keeping them interested requires well-balanced and open communication on both sides.
The hardest time to win investors over is when you are going down into the “disillusionment” trough. Having a strategy lined up for your funding rounds before this point will make your journey smoother. – Pieter de Wilde
Here are some suggestions for funding your project:
1) Aim for a sizeable investment early in your development project. Most likely your seed investment money will only cover the early hype period. But planning and having sizeable series A/B funding strategies is key to survive and cover the trough. The timely availability of mock-ups or prototypes can be of big help here.
2) Government funding may be available for new technologies. Many options are available on the local, regional, national and European level. For example, the European Commission has put €95.5 billion into research and innovation funding through Horizon Europe, which is available for businesses of all sizes. Many countries offer similar support through national programs.
3) Get your medical devices into a clinical trial. Find a collaborator who is willing to integrate your medical device into their application or research program. It could benefit their own research and provide you with free, but essential, clinical data. Also, if early versions of a product are not ‘put on the market’ but sold to academic hospitals for example, they need not to be CE marked and can still provide early revenue.
4) Break your medical device into parts. If your technology is modular or usable in parts, then it may be worth marketing part of the product ahead of the full device – for example, a disposable component. This can provide you with early revenue, and investors are more willing to invest in something that has already shown value.
Regulatory considerations
This has an impact on how the external world views your device and the willingness to integrate it into their own ecosystem. Three important actions can help you in your regulatory trajectory:
1) Get your ISO13485 certification as soon as possible, but be smart. If you have proven your technology, make it a priority to get this done in a pragmatic way. It will sharpen the strategic discussion on what kind of organization you want to be. Having this certification will also guide the collaboration with your R&D partners, making quality tasks and responsibilities clear for all project stakeholders, including your own team.
2) Get your CE mark. This is less time-critical than obtaining your ISO certification, but it is essential in your planning as it is linked to risk mitigation for investors and creates opportunities for unplanned clinical trials. There are many trials that you can take part in without having a CE mark, so you can still make valuable progress before you get to this stage.
3) Align your regulatory trajectory with your technological development. In many cases, regulatory support comes too late, with consequences such as a delayed launch or development phases that need to be repeated. Small technology changes can have a large impact on your risk assessment.
From the start of the development of a new medical device, a solid regulatory trajectory should be projected on top. Every choice made in the former has a consequence on the latter, and vice versa.
Technology considerations
When developing new medical devices, it would seem obvious to put the technology central. After all, it’s the core of your product! Nevertheless, there are aspects that are less evident but that could greatly streamline the path towards innovation:
1) Define your minimal viable product. This is a simple version of your product with just enough features to be usable, getting it the right attention from investors, future users, or distributors. Getting non-functional mock-ups in time helps in doing timely usability studies, and functional prototypes can serve your innovators or early adopters.
2) Define your use case well from the start. Simplifying your process starts with knowing which technologies you do and don’t need in your device. Overcomplicating your device with technology can create unnecessary risks for your project, like excessive cost-of-goods. Investing the time in building a use case with your preferred end user can provide you with valuable feedback, and help you to build an ecosystem around your project.
3) Map out the full life cycle. Moving from early research and development to final manufacturing takes time. Although it might change throughout the process, it’s important to have control over the device’s life cycle early on. The choice of components, their secured availability, the solidity of the manufacturer, their innovative character: these will greatly influence the success and successful marketing of your final product.
Seek experts to manage your medical device development hype cycle
Mapping out and managing your hype cycle is not something to do alone. Partnering up with experienced and complimentary service providers will greatly boost your chances of success.
Read the full white paper here: www.unitron.nl


