Beyond gluten-free: a hopeful future for those who suffer from Celiac disease

Gluten-free dietary products contain more sugar and fats but fewer nutrients and are generally more expensive. A gluten-free diet can even lead to social isolation and stigmatization. Scientists are thus looking for a way that allows people with celiac gluten intolerance disease to enjoy the benefits of gluten in a safe manner.
Looking forward: Azalea Vision’s smart lens is a game-changer for ocular disorders
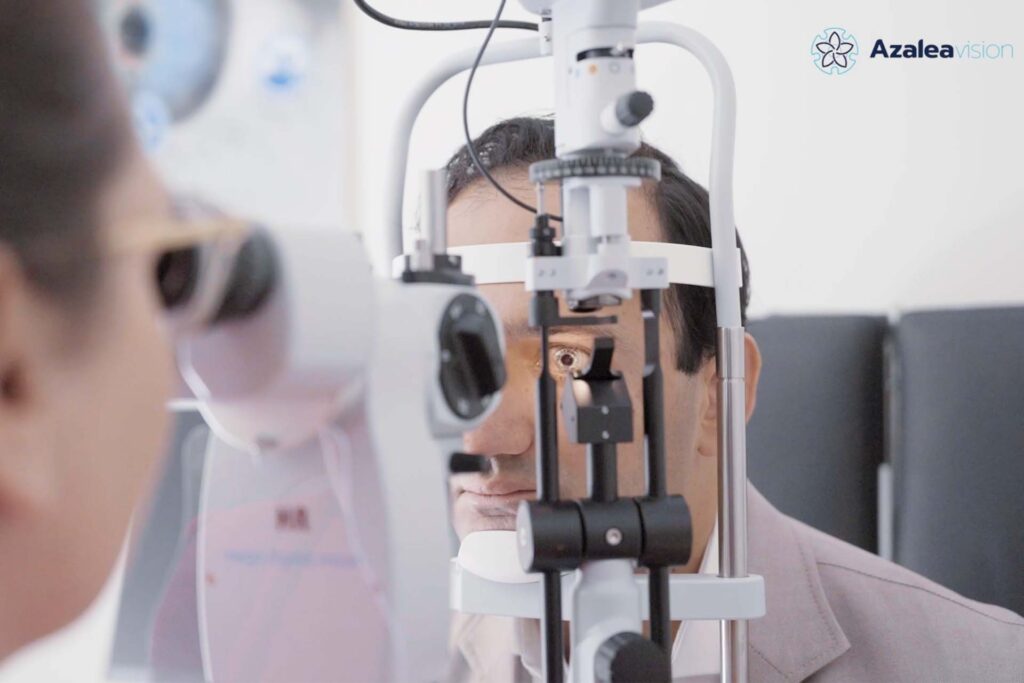
In December 2023, Azalea Vision announced the first test of their smart contact lens on a real person. This demonstration showcased the first functional prototype of their smart lens, known as the ALMA lens, which was developed by the company to address ocular disorders characterized by the inability to effectively filter light.
V-Bio Ventures-funded Tanai Therapeutics develops first-in-class therapeutics for obesity

VIB, Flanders’ leading life sciences institute, together with lead investor V-Bio Ventures has launched a new spin-off company based on research at the VIB-UGent Center for Inflammation Research. With an initial seed financing round supported by V-Bio Ventures, Qbic and VIB, the spin-off company is entering the obesity space with an entirely novel therapeutic approach.
Obulytix is developing a revolutionary solution for antibiotic resistance

Obulytix, a spin-off based on research results from Ghent University and KU Leuven, has built a platform that creates new ways to tackle bacterial infections. The innovative, phage lysin-based platform – leveraging the power of artificial intelligence – attracted a significant four-million-euro investment from Boehringer Ingelheim Venture Fund, Qbic, and Gemma Frisius Fund.
Azalea Vision announces first on-eye test of ALMA, its revolutionary smart contact lens

In a significant milestone, Azalea Vision, pioneering start-up on a mission to revolutionize the treatment of ocular disorders, announces the successful demonstration of ALMA Lens, the first functional prototype of the Azalea smart contact lens’ platform.
miDiagnostics announces collaboration with Galapagos to develop closed-flow, ultra-rapid CAR-T sterility test for its point-of-care CAR-T manufacturing platform

Leuven, Belgium, November 10, 2023 – miDiagnostics NV, a global technology leader in point-of-care diagnostics, today announced a collaboration agreement with Galapagos to develop a closed-flow, ultra-rapid PCR sterility test for CAR-T batch release in a decentralized manufacturing setting, in compliance with regulatory requirements, including those in the U.S., Europe and Japan. Under the terms […]
V-Bio Ventures portfolio company Agomab raises $100 million Series C to advance fibrosis-focused pipeline

Agomab Therapeutics NV (‘Agomab’) today announced the closing of a $100 million (€94.9 million) Series C financing round led by Fidelity Management & Research Company, with participation from new investors EQT Life Sciences (EQT), Canaan, Dawn Biopharma, a platform controlled by KKR, and existing investors.
Pharmaceutical market access: how real-world data fuel better health beyond clinical trials

Studies of a drug’s effectiveness and safety don’t end with clinical trials – they extend beyond market access when the true value of a drug is demonstrated in a real-world setting. Based on this information, factors such as availability, pricing, and reimbursement are adjusted. To study the actual worth of a treatment, we require real-world data (RWD) from a large and diverse patient population. By actively sharing this information with stakeholders, we can fuel further research and innovation, and even help to inform decision-making on a population level. But in order to unlock the full potential of our patient data, all members of the ecosystem have to work together.
V-Bio Ventures portfolio company Orionis Biosciences announces collaboration with Genentech to discover and develop molecular glue class medicines

September 20, 2023, BOSTON, MA and GHENT, Belgium – Orionis Biosciences, a privately held life sciences company with an integrated drug discovery and chemical biology platform, announced today a multi-year collaboration with Genentech, a member of the Roche Group, to discover novel small molecule medicines for challenging targets in major disease areas, including oncology and […]
Augustine Therapeutics expands its leadership team and R&D platform, enters regulatory development.

Leuven, Belgium, September 12th, 2023 – Augustine Therapeutics, a biotech company developing innovative therapies for neuromuscular and neurodegenerative disorders, announced today the expansion of its leadership team with Marie Trad, MD as Chief Medical Officer, Jonathan van Eyll, PhD as Head of Pharmacology and Cédric Szyndralewiez, MSc as Head of Non-Clinical and Early Development. The […]
V-Bio Ventures portfolio company Corteria Pharmaceuticals raises EUR 65 million to advance its transformational medicines for cardiovascular diseases

Ghent, Belgium, 7 September 2023 – Corteria Pharmaceuticals, a V-Bio Ventures portfolio company, is a biopharmaceutical company specialized in the development of transformative therapies for unaddressed heart failure subpopulations. Corteria today announced an oversubscribed EUR 65 million Series A co-led by US investment firm OrbiMed and EU-based leading investment firm Jeito Capital, with the participation […]
The obesity revolution: will new drugs bring about big changes?

The recent approval of the obesity and diabetes drugs Wegovy, Ozempic, and Mounjaro has brought about big headlines, but are these treatments also leading to a shift in our perception of obesity? Obesity has long been seen as a failure of individual willpower, but it is becoming increasingly apparent that losing and keeping off the kilos is a more complex, biological challenge. These new drugs offer a first, rather simplistic solution to a complicated problem, yet perhaps they will lead to a much-needed revolution in how obesity is viewed and treated.
V-Bio Ventures portfolio company Dualyx raises €40 million to progress Treg therapies for autoimmune diseases into the clinic

Use of proceeds will enable the development of the Company’s lead autoimmune program DT-001, targeting TNFR2, as well as its pipeline of Treg candidates Financing co-led by Fountain Healthcare Partners, Forbion and Andera Partners with support from existing investors Bernard Coulie appointed as Independent Chairman with immediate effect Ghent, Belgium, 15 May 2023 – […]
Corteria: a new strategy and approach to treating heart failure

Corteria Pharmaceuticals is developing transformative therapies for heart failure subpopulations most likely to benefit from the company’s novel cardiorenal treatments. The company was founded in 2021 by Sanofi’s former head of cardiovascular research, Philip Janiak, and Marie-Laure Ozoux, former cardiovascular project leader at Sanofi.
Data science: empowering animal-free progress in preclinical development

Many drugs fail clinical trials, often because preclinical animal models fall short of replicating human physiology. To improve animal welfare, speed up drug development, and reduce costs, we need to rely less on animal models, while also minimizing the number of failures early in the drug development process. Artificial intelligence and machine learning are powerful tools that can help us achieve these goals by predicting a drug’s efficacy, safety, and uptake in preclinical studies. These technologies can help researchers to make informed decisions and optimize testing strategies, improving drug development for both animals and people.
