The American investor and philanthropist Warren Buffet once said: “No matter how great the talent or efforts, some things just take time. You can’t produce a baby in one month by getting nine women pregnant.”
The arduous biotech trek – from the inception of a groundbreaking idea to the successful launch of a product – reflects this concept. Some things simply can’t be rushed. Biotech companies face a long, highly-regulated and capital-intensive path to bring their innovations to life. However, the effort is incredibly worthwhile, as these innovations may have a profound and long-lasting impact on healthcare and human lives.
Discovery and early research: the birth of an idea
The journey of a biotech startup begins in the lab, often driven by academic research or cutting-edge discoveries in fields like molecular biology, genomics or bioengineering. At this early stage, scientists and researchers explore novel concepts such as identifying new drug targets, developing groundbreaking therapies or designing advanced diagnostic tools.
This research typically relies on grants from government institutions, universities or research foundations, as there is scant interest from private capital in financing research activities before ‘Proof of Concept (PoC)’ has been achieved. At this early point in the biotech life cycle, it’s vital for company founders to secure patents and IP protection – strong IP can be a cornerstone for attracting future investments and establishing a competitive edge.
Preclinical development: moving from idea to prototype
Once a promising academic breakthrough has been made, there are many steps needed to turn that discovery into a viable product or therapeutic candidate. The first stage is usually preclinical development, which involves testing the concept in laboratory settings (typically through cell assays, animal models or patient sample studies), assessing the product’s safety, efficacy and mechanism of action.
A strong scientific team is pivotal at this stage to drive the company forward and handle the countless unforeseen issues that are inherently linked to a biotech project. It’s helpful for companies to have a clear ‘Target Product Profile (TPP)’, as it provides a roadmap for keeping the program on track and ensures the future product to be commercially viable.
Often, a startup will need to go through several optimization steps before it’s able to obtain a first proof of concept in a biological system that is relevant and predictive for patients. This is a critical step that validates the initial idea and attracts the interest of investors, including venture capital firms.
In parallel, many biotech startups also leverage government grants, academic partnerships and industry collaborations to obtain non-dilutive funding. Other key inflection points – often pursued in parallel – are the preparatory steps necessary for the approval of future human clinical trials, including ‘Chemistry, Manufacturing and Control (CMC)’, pharmacology and toxicology studies.
Clinical trials: the road to validation
If a company is able to obtain an extensive data package in its preclinical phase, it can build on that strong foundation to move forward into clinical trials. This clinical stage is one of the most important milestones for a biotech startup. It involves human testing and is typically divided into three phases, each associated with a steep increase in costs:
- Phase I focuses on safety, testing the product in a small group of healthy volunteers (usually 20 to 80 participants).
- Phase II expands testing to a larger group (generally 100-300 patients), to assess efficacy and side effects.
- Phase III involves large-scale trials (often including thousands of patients), to confirm efficacy and monitor the product for long-term side effects.
At this advanced stage, regulatory affairs become critical for a biotech company, as it must secure approval for each clinical trial from the relevant regulatory body. A mistake might lead to significant trial delays and subsequent cash burn, which is especially problematic as clinical trials are already extremely expensive and typically take multiple years to complete.
Most of a company’s value inflection points happen during this critical stage. Despite having the lowest probability of success, Phase II is often regarded as the most significant value-adding step in a company’s journey, as it involves establishing ‘Human Proof of Concept’. If that is achieved, a company’s potential skyrockets, meaning that there is a huge opportunity for venture capital firms who are willing to take a risk and invest before this Phase II milestone. Most biotech merger and acquisition deals also take place around this time, as larger biotech or pharmaceutical companies make educated guesses on a startup’s chance of success.
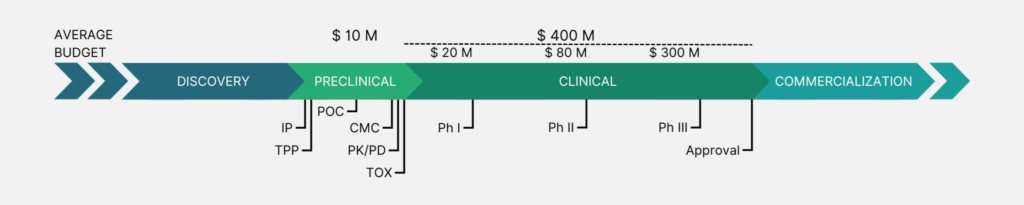
The last steps: approval, commercialization and expansion
If clinical trials are a success and the startup decides to proceed with its own in-house development, then the next step is to seek market approval for its product from regulatory authorities like the FDA (U.S.) or EMA (Europe). This process involves submitting extensive data to demonstrate that the product is safe and effective for its intended use. Companies that successfully navigate the regulatory approval process – especially in emerging therapeutic areas – can gain a first-to-market advantage, securing a significant market share and attracting further investments.
The next major step is bringing the product to market. Commercialization involves scaling up manufacturing, creating distribution channels and initiating marketing and sales. Market adoption requires extensive efforts to convince healthcare providers, patients and insurers that the new product will benefit them. As this is a tough challenge, the vast majority of startups choose to collaborate with larger pharmaceutical companies during this stage.
Finally, if the launch of its first product is a success, then the biotech company will need to look towards the future. To secure long-term success, it will need to expand its product pipeline – maintaining innovation through continuous R&D efforts and/or business deals – and continue to fuel its ambitious growth trajectory.
The high-risk/high-reward journey of biotech startups
From lab to market, the life cycle of a biotech startup is a long, complex and capital-intensive process. However, the potential financial and societal rewards are significant. Biotech startups have a unique opportunity to transform healthcare through true innovation, addressing some of the world’s most pressing medical challenges.
At V-Bio, we strive to support our portfolio companies through the early stages of this complex endeavor, relying on our familiarity with the common patterns and pitfalls, to provide helpful guidance and advice. For both investors and entrepreneurs, a solid understanding of the biotech life cycle is fundamental to navigating the risks and seizing the best opportunities at each step of the biotech journey, working together to achieve long-lasting impact on global health.


