ASIT Biotech believe they have the answer to our sneezing and itching: a short-course treatment to protect you from this vicious annual onslaught of grass globules! The Belgian company, which raised €13.9Mil earlier this year, is hoping to have its hay fever treatment on the market by 2020. Currently the product is undergoing a second round of Phase III clinical trials. Competition is fierce; with the global allergy market expected to grow to €33.35Bn by 2025, ASIT Biotech is vying for its market share with several others, including the UK based Allergy Therapeutics. BioVox spoke to Thierry Legon, ASIT Biotech CEO.
Sunshine and sneezes
What are the current options for treating hay fever and what is ASIT Biotech doing differently?
Legon: “To treat respiratory allergy today, your main option is to use symptomatic drugs, such as antihistamines and corticosteroids. This is often enough for patients who only suffer from mild or infrequent allergic reactions, but for those who suffer more severely there is an alternative path: immunotherapy. The problem with current allergy immunotherapy is that the courses are far too long. You have to see a doctor a minimum of 40-60 times over a period of three years. With such prospects, a lot of people refuse to even start treatment and the majority who do begin a course will not see it through to completion.”
We believe that if we can provide short-course treatments, patients will be much more eager to use immunotherapy to eliminate their allergies altogether and live a better life.
“At ASIT Biotech, we are developing a much shorter immunotherapy course, to improve patient acceptance and compliance with allergy treatments. With our short-course, you will only need to attend an allergist’s office four times over a period of three weeks. It is a bit like a flu vaccine: every year, before the pollen season, you get your shots and you are protected until the end of the following summer.”
Apart from the crowd
How does the ASIT technology work?
Legon: “Our treatments work by extracting and purifying natural allergens from one grass species responsible for most hay fever cases. We break the allergens down into fragments, to reduce their potency to trigger an allergic response. We use fragments of varying lengths in order to activate both B-cells and T-cells, triggering the immune system in its optimal complexity.”
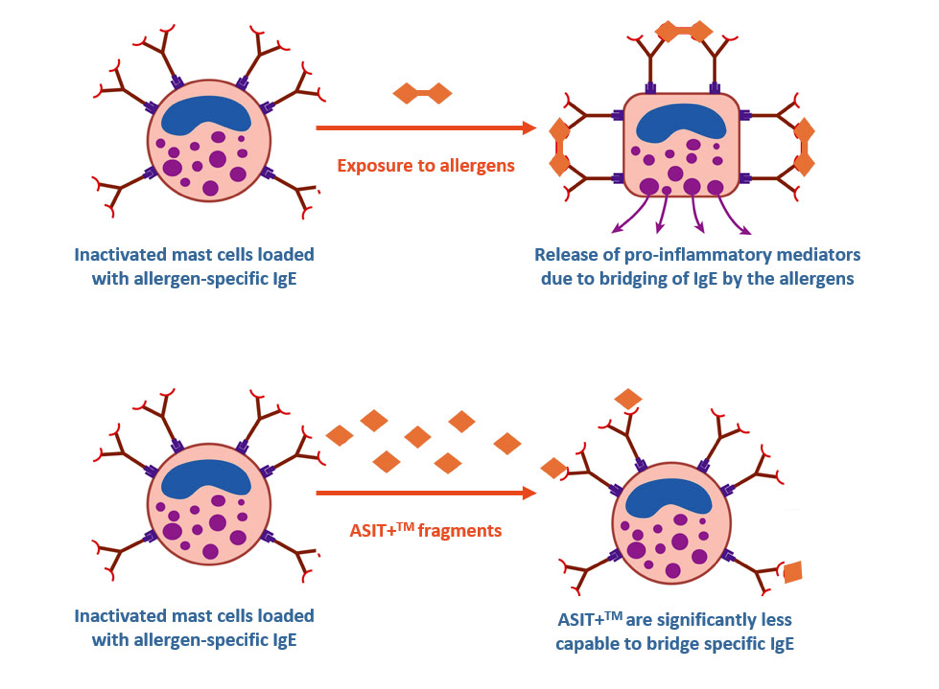
ASIT technology: Natural allergen fragments bind to antibodies in a way that is less likely to result in an inflammatory response (sourced from ASIT Biotech website).
“Our product lies somewhere between synthetic peptides and whole allergen extracts; both methods have been used by various companies in the past: Circassia used small synthetic peptides in their products that only activated T-cells. They scrapped their whole allergy pipeline in 2017 due to several failed Phase II trials. Allergy Therapeutics use whole allergens in their trials but combine them with adjuvants (substances that enhance the body’s immune response to an antigen). I believe adjuvants can be useful: if you are protecting a patient against infectious disease or cancer, for example. However, for allergy treatments I don’t believe the safety/efficacy balance is in favor of using potent adjuvants. ASIT products are adjuvant-free because we believe that it will reduce long term toxicity and the potential unwanted side-effects.”
The problem with current allergy immunotherapy is that the courses are far too long. You have to see a doctor a minimum of 40-60 times over a period of three years. With such prospects, a lot of people refuse to even start treatment and the majority who do begin a course will not see it through to completion.
Can the ASIT technology be used to develop treatments for any other forms of allergies?
Legon: “Yes, it can: the technological platform we have developed can be applied to many different types of substances, including food allergens (like peanut, cow’s milk and egg white) and other respiratory allergens (such as house dust mites and ragweed). Immunotherapy is designed for patients with moderate to severe allergies, who are not satisfied with symptomatic drugs. We believe that if we can provide short-course treatments, patients will be much more eager to use immunotherapy to eliminate their allergies altogether and live a better life.”


