In the 1980s, Dr. Cantley’s team discovered a key type of enzymes called phosphatidylinositol 3-kinases (PI3Ks). These enzymes, which promote cell growth and division, produce phospholipids whose existence had been overlooked for almost 35 years. As it turns out, changes in the PI3K pathways are responsible for causing the majority of known cancers in humans. Drugs targeting these enzymes have proven more effective and less disruptive treatments for cancer, with several PI3K inhibitors now on the market and saving lives. We had a chat with Dr. Cantley about the discovery and his personal motivators for conducting research.
Can you tell us a bit about your background and why you became a researcher?
“My father was very science driven and was particularly interested in weather. So, growing up in rural West Virginia, I would ask my father questions like ‘why does it rain?’. Where my friends’ fathers would say something along the lines of ‘God makes it rain so we can have vegetables’, my father would instead go into great detail about how particles form and allow rain drops to fall. My father’s responses to these types of questions initiated in me a passion for understanding how things work at the molecular level.
Then in high school, I fell in love with chemistry. Of all the subjects I took, I thought it was the most simple: chemistry is very visual, and once you know the rules, the rest is pretty straight forward. That really appealed to me.
I got my undergraduate degree in chemistry and math and then went to Cornell for my PhD in biophysical chemistry. That’s when I first got introduced to biological research. From there, I went to Harvard for a post doc and eventual assistant professor’s position, where the breakthrough with PI3Ks happened.”
How did your discovery of PI3Ks come about?
“In the 1980s, my lab was working on membranes and trying to understand lipids. It had been known for quite some time that a lipid called phosphatidylinositol, which makes up a part of the inner membrane layer of cells, was being phosphorylated. By strange coincidence, the discovery of phosphatidylinositol phosphorylation was made in the year I was born. However, for almost 35 years, it was thought that phosphatidylinositol only had phosphate groups added in the 4 and 5 hydroxy position of the inositol ring. In the late 1980s, we discovered that there was in fact another type of lipid kinases that were phosphorylating phosphatidylinositol in the 3 position. We named them Phosphoinositide 3-kinases, or PI3Ks.”
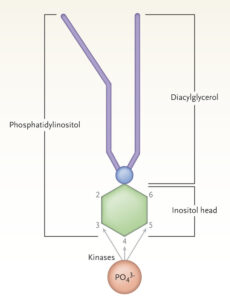
The molecule at the center of it all: Phosphatidylinositol. It consists of an inositol molecule, linked to glycerol and two fatty acid tails via a phosphate group on the 1 position. Because of steric hindrance, phosphorylation (the addition of another phosphate group) is only possible on positions 3, 4 and 5. Until Cantley’s discovery, it was thought that only 4 and 5 could be phosphorylated. The PI3K enzyme family, enzymes capable of phosphorylating the hydroxy group on position 3, opened up the exploration of previously overlooked phospholipids that are vital to a wide range of biological functions. PI3Ks are also important in a wide range of diseases, including diabetes and cancers (image courtesy of Lewis Cantley).
“Our discovery resulted in a whole new family of lipids being uncovered. The field suddenly exploded, with discoveries of new molecules that were vital for insulin regulation of blood glucose levels, cell growth and, as it turns out, diabetes and cancers.”
When your discovery was first published, there was a lot of skepticism from the academic community. Why was that?
“Yes, there were a lot of people who actually refused to believe our results. People who couldn’t accept that they had failed to notice these important lipids for 35 years. One of the naysayers even said: ‘If this turns out to be true, I will eat my hat’!
There were a couple of reasons PI3Ks had been missed. One was that telling the difference between a lipid with a phosphorylated 3 or 4 position was really hard, because the structures are so similar. Another reason was that phosphorylation at the 3 position only happened if you added a growth factor to the cell. So if you looked at tissues that weren’t growing, you would essentially not be able to detect these lipids.
On top of that, in the years following our discovery there were actually several papers published saying that our results were not reproducible. It was frustrating: the people that were making these complaints were Nobel Laureates and leaders of the field, so these papers prevented me from getting grants for a few years.
There was never any doubt in my mind that I was right though, and I knew they would eventually figure it out. Because I knew exactly why they weren’t getting the results we had. Many of these researchers were from molecular biology backgrounds rather than from biochemisty: they were cancer researchers, not enzymologists or biochemists. Because of my background in biochemistry, I realized that you had to look at phosphorylation of these lipids in a membrane compartment, because that’s the way it naturally occurs. So to study the phosphatidylinositol phosphorylation event, we made a synthetic membrane which mimicked what you would see in a cell. None of the laboratories trying to replicate our results were doing that; they just dissolved everything in detergent. Eventually, one of my graduate students actually flew to these various laboratories to personally show them the synthetic membrane technique. After that, these labs finally published papers supporting our results. I never did find out if that one detractor ate his hat.”
What further discoveries has your research led to?
“By the early 1990s, I realized that there must be a link between PI3Ks and cancer. Bert Vogelstein’s work [for which he won the Dr. Paul Janssen Award in 2015] supported this, when his lab discovered that PI3Ks are very frequently mutated in cancers. In fact, this pathway is implicated in about 80% of cancers. It is the number one mechanism by which tumors occur.
Yet PI3Ks are also closely involved with insulin and therefore with diabetes. Insulin resistance is essentially a failure of insulin to activate PI3K. So the pathway is a double edged sword: if you can’t activate PI3K, you end up with diabetes. If it is over-activated, it can result in cancer. It’s a fine balance. This is why my lab studies both of these fields, because this same pathway continually allows us to understand both diabetes and cancer and therefore to find potential solutions to both.”
How has your research been applied and what impact has this had on people’s lives?
“My discoveries have led to both a better understanding of biology, but also to multiple therapies. My work with PI3Ks in cancer has by now led to the development of several new drugs based on PI3K inhibitors. I’ve personally been involved in the clinical trials for a few of these, and there are now several on the market that are already saving lives. The first PI3K inhibitor to be approved was idelalisib [by Gilead Sciences, approved in 2014]. That was followed by copanlisib [by Bayer, approved in 2017], duvelisib [by Verastem Oncology, approved in 2018]. All three are used to treat different types of blood cancers. The first PI3K inhibitor drug to be approved for solid tumors was alpelisib [by Novartis, approved in 2019], which is used to combat breast cancer. I think we will be seeing many more PI3K-based treatments in the future, as we uncover more about the clinical potential in this field.”
Read this previous BioVox article for more on the winners of the Dr. Paul Janssen Award 2019: Franz-Ulrich Hartl and Arthur Horwich
The Dr. Paul Janssen Award is part of Johnson & Johnson’s Champions of Science initiative to fuel public engagement, support and trust in science. What’s your message for the next generation of scientists?
“You have to be curious, you have to be brave, and you have to be willing to share your ideas. Don’t be secretive; work with others to explore your questions. And have fun! To me, science is a curiosity-driven pursuit to deeply understand how things work.
I think this kind of attention to detail and good biochemistry is important for laying the foundations for future science discoveries, in the same way that my basic discovery paved the way for new cancer drugs. You need to do good basic science to get to the bottom of things. Because once you understand exactly how something works then you can fix it. So, I think that is how you should approach your research: break it down and understand the basic parts and how it all fits together.”


