Sepsis is a life-threatening condition caused when the body’s response to an infection injures its own tissues and organs. Sepsis affects 49 million people globally each year, resulting in 11 million deaths and accounting for 1 in 5 deaths worldwide. Sepsis is now recognized as a global health priority by the World Health Organization. Unfortunately, treatment options are lacking as the contributing factors remain poorly understood. Our research group, under the supervision of Professor Claude Libert (VIB-Ghent University), is on a mission to understand the mechanism(s) leading to sepsis. Our ultimate aim is to transform sepsis into a disease that is no longer a leading cause of death worldwide. We focus on the role of a well-known cytokine called Tumor Necrosis Factor (TNF) in sepsis. Cytokines are signaling proteins that facilitate cell-cell communication to help the body fight infections, control inflammation, and heal.
TNF – the devil in sepsis
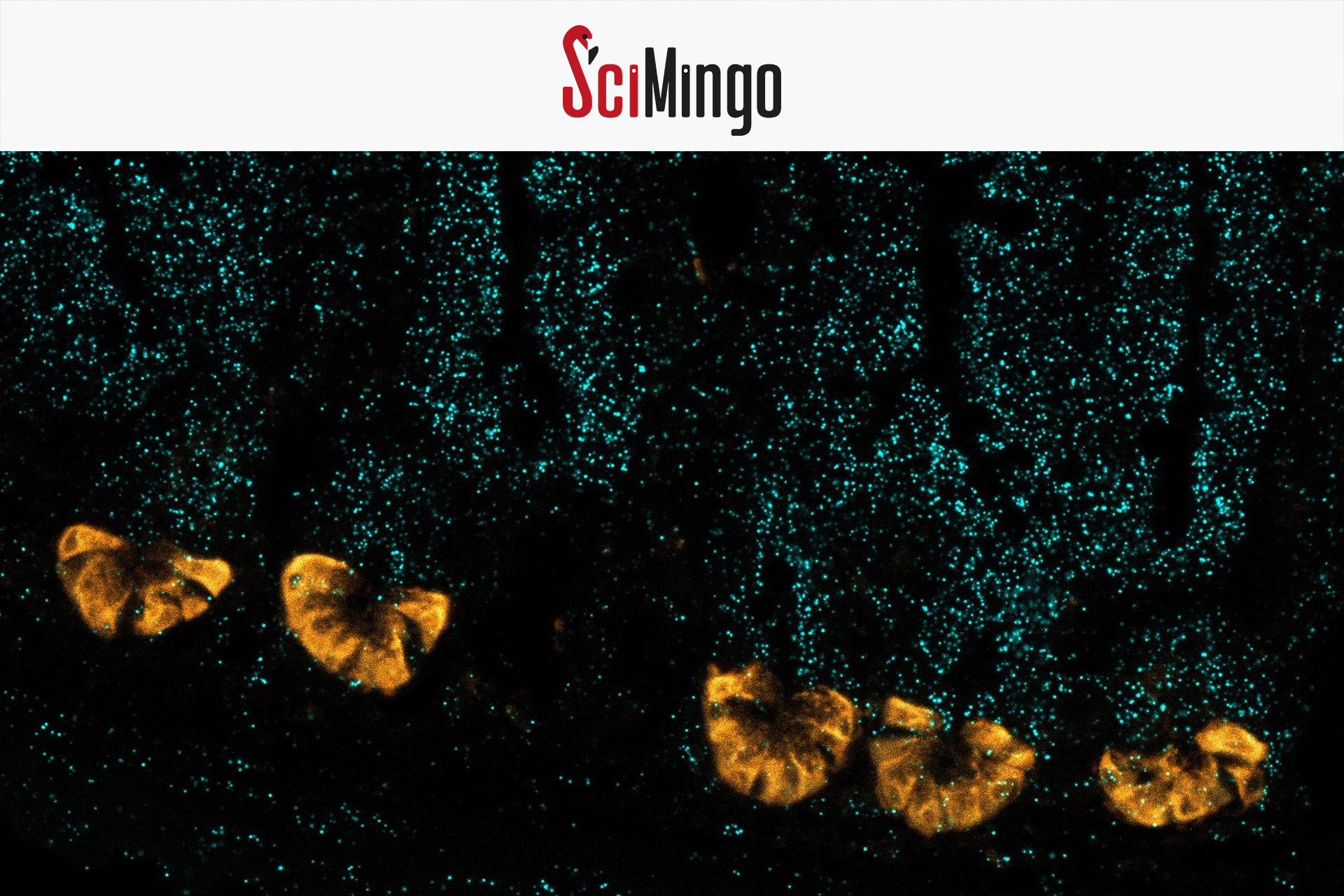
TNF is an important signaling molecule in sepsis. Injection of TNF in mice induces typical sepsis features, but the precise underlying mechanism remains unclear. In our study published in 2024, we uncovered that TNF affects a unique cell type in the intestinal lining called Paneth cells – responsible for the production of antimicrobial proteins. Too much TNF signaling can suppress their antimicrobial activity, allowing bacteria to cross from the gut into the bloodstream and organs, potentially leading to sepsis. Using genetically modified mice that lack the TNF receptor on Paneth cells, we prevented TNF-induced organ damage, showing these cells are key.
Although far outnumbered by other intestinal cells, Paneth cells have a crucial role in maintaining a healthy microbiome and preventing bacteria from crossing the gut barrier. The critical role of TNF’s action on Paneth cells is spectacular, given the low amount of Paneth cells found in the gut.
UPR – the angel of defense
To dig deeper, we then discovered that TNF’s devilish effect on Paneth cells happens because it disrupts the Unfolded Protein Response (UPR). The UPR ensures that proteins are folded properly to function correctly. Our findings reveal that Paneth cells have an exceptionally high UPR activity to meet their elevated protein production demands. By attacking the UPR, TNF however perturbs the production of antimicrobial proteins and hence UPR’s antibacterial activity in Paneth cells.
Key insights and lessons learned
In the past, clinical trials using anti-TNF therapeutics failed, possibly due to the oversight of TNF’s multifunctional role across virtually every cell type. For example, TNF has an essential role in immune cells by initiating an immune response against invaders. Unlike prior studies that focused on global TNF inhibition, our work pinpoints a specific target—Paneth cells—offering a precise and potentially safer therapeutic approach.
TNF seems to be a causative agent triggering sepsis, not merely a consequence. Inflammatory diseases with high TNF production in the gut, such as inflammatory bowel disease and coeliac disease, might thus increase the risk of sepsis.
This research brings us one step closer to therapies that can save millions of lives. Future research will be needed to turn our findings into actionable treatments that patients desperately need.
Header image: Paneth cells by (c) Charlotte Wallaeys