The UCB core drug CIMZIA® has just been approved by European Medicines Agency (EMA) for use in pregnant and breastfeeding mothers. The company has also sought to have CIMZIA approved for psoriasis treatment, as it is currently only indicated for rheumatoid arthritis, psoriatic arthritis and axial spondylitis. Biovox spoke to Emmanuel Caeymaex, Head of Immunology and Executive Vice President at UCB, regarding the CIMZIA reclassification, as well as another psoriasis drug in the UCB pipeline: bimekizumab.
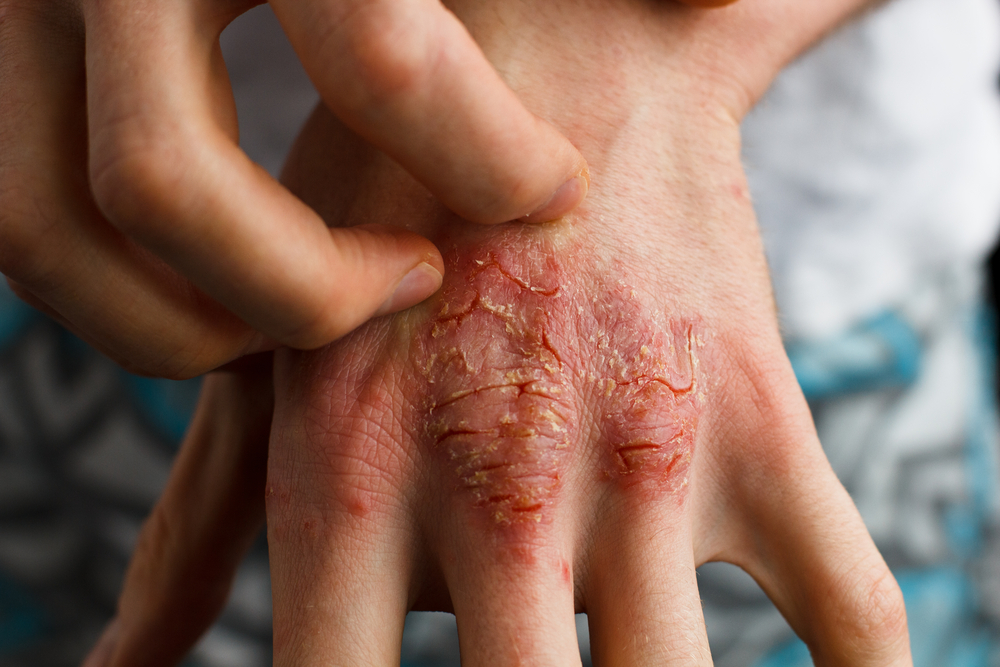
Psoriasis is a common, immunological disorder affecting nearly three percent of the world’s population. For most of the 125 million people affected by psoriasis, the disorder presents as a dermatological issue: an overgrowth of skin cells leads to red patches with silvery scales, and dry, cracked, even bleeding skin. The condition affects both men and women, but for women of childbearing age treatment options have until recently been limited.
Tell me about CIMZIA: how does it work and what is it used for?
Caeymaex: “CIMZIA is a biotech product that neutralizes the tumor necrosis factor (TNF), a cytokine that is important in a range of autoimmune disorders. It is a form of symptomatic treatment: it helps reduce one of the key drivers in the inflammatory cascade. Until now, CIMZIA has only been indicated for rheumatoid arthritis (RA), psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA).”
When did you start investigating if CIMZIA could be used for psoriasis?
Caeymaex: “We actually started in 2010: we got some insights from the marketplace that the efficacy of existing psoriasis drugs often wanes after six months to a year. We understood that there was an opportunity to develop a TNF inhibitor that provides more sustained efficacy over time.
We also embarked on this journey to generate evidence for women of childbearing age; to determine whether CIMZIA crosses the placental barrier during pregnancy. Other therapeutic antibodies in this category do, which is why a lot of physicians recommend ceasing therapy before pregnancy. We see that as a trade-off: you want to protect the baby, but then the mother’s disorder may worsen. We have now documented and proven that CIMZIA doesn’t cross the placental barrier, and what is more: it is only minimally transmitted through breastmilk.”
Even if a drug reduces your body surface impacted by psoriasis by 98%, if the remaining 2% happens to be on your genitals and face, you haven’t solved much from a quality-of-life point of view.
In addition to CIMZIA’s developments, UCB has another psoriasis drug in the pipeline: bimekizumab. The company presented the promising phase II results for the drug at the 2018 American Academy of Dermatology annual meeting last month. We asked Caeymaex about bimekizumab and how it differs from current drugs on the market.
Tell me about bimekizumab: how does it differ from CIMZIA?
Caeymaex: “Bimekizumab is a therapeutic antibody that acts on the interleukin 17 (IL-17) pathway which is associated with several chronic inflammatory diseases. The drug is like a single key that opens two locks, neutralizing both IL-17A and IL-17F, two “sibling” cytokines. Bimekizumab is at an earlier clinical trial stage compared to CIMZIA: phase III for psoriasis patients and we plan to start phase III studies for PsA and axSpA later this year. We are also exploring its effect on a disease called hidradenitis suppurativa, which is a terrible autoimmune affecting mostly women, which only has one product approved for treatment so far.
We are taking bimekizumab forwards because we have established that maximizing the efficacy of an agent in the IL-17 pathway means blocking both IL-17A and IL-17F. Today there are agents that block IL-17A alone, like Cosentyx by Novartis or Taltz from Eli Lilly, but not all patients achieve completely clear skin with these drugs. That is why we have directed our focus to bimekizumab: an agent that blocks both cytokines and therefore clears the skin completely.
What are the next steps for bimekizumab? When might it be available?
Caeymaex: “The phase III is a superiority study so we are evaluating the performance of bimekizumab as compared to currently available drugs. The products we have picked are the two treatments that are currently standard of care: Humira and Stelara. We’ve also picked Cosentyx, which is an IL-17A blocker, to really prove the point that neutralizing both IL-17A and IL-17F makes a significant difference to the treatment outcome. Trials for PsA and axSpA are also about to start.
Given standard development times, you could see bimekizumab submitted to the regulators to be approved for psoriasis in 2020 and launched by 2021. Then maybe in 2022 or 2023 for PsA and axSpA, depending on the trial progression.”
“Total clearance is really the goal in psoriatic treatments now. Even if a drug reduces your body surface impacted by psoriasis by 98%, if the remaining 2% happens to be on your genitals and face, you haven’t solved much from a quality-of-life point of view. The test results from our bimekizumab psoriasis studies are quite impressive: in just 12 weeks you have up to 60% of patients with completely clear skin. That hasn’t been observed before, not at that magnitude. We are confident in the future outcomes of this drug. “