Why do we need healthcare data standards?
Date of admission, diagnostic tests, procedures, patient instructions, lab results, prescriptions – the amount of data hospitals gather and create upon admitting patients is significant, to say the least. However, this wealth of information is currently not being utilized to its fullest potential for understanding disease characteristics and enhancing patient care. The main hurdle in achieving this goal is the inadequate sharing of information between healthcare organizations, clinical laboratories, research centers, disease registries, and other relevant stakeholders.
“Interoperability is crucial in facilitating the reuse of data, which in turn is essential for large-scale research and analytics.”
In order to communicate effectively, both sender and recipient must speak the same language – otherwise messages may be misunderstood, resulting in the loss of valuable insights or the drawing of inaccurate conclusions. The language in which patient data is recorded and shared needs to be agreed upon and, to do so, data standardization is essential. These standards are tools – such as procedures, vocabularies, and requirements – that help structure and represent clinical information in a common format that allows storage, exchange between systems, and access by different healthcare applications. Interoperability – the ability of computer systems or software to exchange and make use of information – is crucial in facilitating the reuse of data, which in turn is essential for large-scale research and analytics.
OMOP vs. FHIR
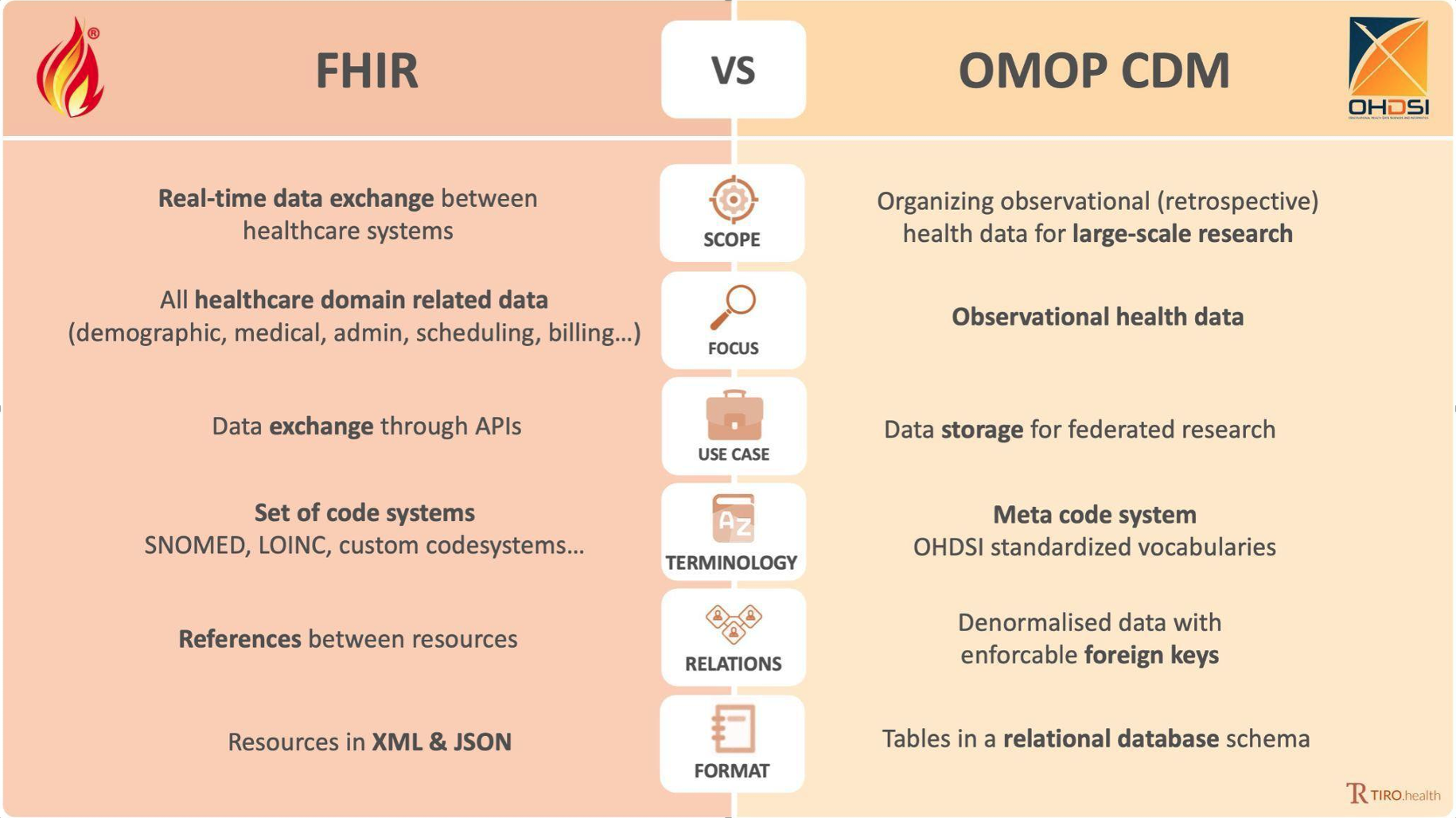
Two well-known standards that are widely used to structure healthcare data are OMOP CDM (Observational Medical Outcomes Partnership Common Data Model) and FHIR (Fast Healthcare Interoperability Resources). These standards are not necessarily competitors: they have different functions and can complement each other to achieve specific objectives.
OMOP was originally established as a 5-year public-private partnership to improve the efficiency and accuracy of post-market drug safety surveillance (a need which was expressed by the U.S. Food and Drug Administration). The resulting standardized approach for structuring healthcare data resulted in a common data model, and further evolved into the establishment of the OHDSI initiative (Observational Health Data Sciences and Informatics) – an interdisciplinary open-science collaborative aiming to generate evidence that empowers better health decisions and improved care.
FHIR was introduced several years later by HL7 (Health Level Seven International) – a global authority on healthcare data interoperability standards. Where OMOP CDM represents a standard for organizing and representing healthcare data for analysis, FHIR is a standard for exchanging healthcare information between different software systems in a way that is fast, easy to implement, and flexible. This allows different systems – such as electronic health records (EHRs), clinical decision support systems, and mobile apps – to communicate with each other.
Read this article to find our more about data science in early drug discovery!
Choosing is losing
The distinction between OMOP CDM and FHIR makes them applicable in different contexts, yet complementary in specific situations. OMOP CDM is mostly used in academic settings, as the format for representing clinical data is optimal for scientific research. In this context, interoperability refers to the ability to share queries with other analysts, rather than any data in itself.
OMOP CDM use case: Individuals suffering from moderate-to-severe asthma are at a higher risk of being hospitalized due to COVID-19. To study the interaction between both conditions, and concomitant outcomes, it is imperative to integrate the extensive data available from research conducted across the globe. In a recent combined effort, patient data from the US, South Korea, and Europe was brought together through standardization to OMOP CDM, which allows institutions to conduct analytics in a distributed or federated manner without sharing confidential clinical data.
FHIR is developed for the exchange of the healthcare data itself but is less fit as a clinical data storage model. It can cover a broader range of information within healthcare organizations as it integrates clinical information but also information for administrative, scheduling, and billing purposes. Interoperability in FHIR refers to the exchange of the FHIR resources themselves.
FHIR use case: Healthcare apps have gained widespread popularity as a means of monitoring an individual’s health status, physical activity, and wellness goals. The real advantage of these systems lies in their ability to integrate health information with data from other sources (such as EHRs) and share it with authorized healthcare providers. To facilitate this communication, a standardized method for exchanging the tracked information is required. Apple’s Health app utilizes FHIR to integrate and exchange the tracked data.
By leveraging their distinct strengths, the combination of OMOP CDM and FHIR can be a fruitful approach to attaining targeted data management objectives, such as integrating clinical data from different systems into a common data repository for research purposes. In 2021, it was announced that HL7 and OHDSI will collaborate with the aim of creating a unified data model that can facilitate the sharing of clinical care and observational research data. This endeavor is currently still ongoing.
Similar to a handyman in need of a toolbox with both versatile and specialized instruments, healthcare organizations and researchers may need a combination of FHIR’s adaptability and OMOP’s uniformity to accomplish different healthcare objectives and extract meaningful insights from healthcare data.
Interested in learning more about the use of FHIR and OMOP data models in clinical contexts? Find out more about the topic here.