An urgent call for research
An urgent call for research
A staggering 70% of the female population experiences at least one episode of a vaginal yeast infection, also called vaginal candidiasis, in their lifetime. Between 5 and 10% of them suffer from four or more episodes per year, imposing a significant physical and emotional burden. Infection is characterized by a local overgrowth of fungal cells, leading to inflammation and symptoms such as vaginal irritation (including itching, burning, and pain) and a thicker vaginal discharge with a distinct smell. Although not yet completely clear, certain factors such as the intake of oral antibiotics, hormonal changes, and certain lifestyle, hygienic, and clothing habits, appear to increase the chance of infection. The risk of recurrence, at least to a certain degree, seems to be geneticically determined.
Previous research has provided a number of treatments that can help the body to control or eradicate these infections. They are either formulated as an oral tablet or a cream that needs to be applied vaginally, and both seem to work effectively. However, a number of disadvantages are associated with these therapies. First and most importantly, despite their ability to cure an acute infection, the current treatments don’t seem to prevent the infection from returning after a period of time. Women experiencing recurrent infections are thus left without long-term solutions. Secondly, topical therapies often require an extended treatment time of over one week, impacting the quality of life of patients. Thirdly, long-term usage of some of the available therapies can come with side-effects. Finally, some Candida variants, such as Candida glabrata, do not respond very well to regularly-used treatments and need alternative drugs. Unfortunately, research into novel and better treatments to combat these infections is rather limited. “Although some new therapies are being developed at the moment, we do not know nearly enough about these infections to develop drugs that can effectively and safely eradicate them in all patients,” says Mark Gresnigt, Group Leader – Adaptive Pathogenicity Strategies at the Leibniz Institute for Natural Product Research and Infection Biology – Hans Knöll Institute (Leibniz-HKI) in Jena, Germany.
Another issue relates to infection diagnosis. Instead of seeking professional help, women experiencing symptoms such as vaginal irritation often self-medicate with over-the-counter drugs. The main problem here lies in the fact that different vaginal infections often present similar symptoms. Improper diagnosis can result in ineffective treatment and potentially even agravate the problem, especially when antibiotics are used. Accurate diagnosis is absolutely essential, yet even healthcare professionals sometimes struggle to properly identify fungal infections. More research is needed to provide biomarkers that facilitate more efficient and effective diagnoses.
“Although some new therapies are being developed at the moment, we do not know nearly enough about these infections to develop drugs that can effectively and safely eradicate them in all patients.” – Mark Gresnigt, Leibniz-HKI
The need to replace animal models
Historically, two types of animal models have been used in vaginal fungal infection research. In both the rat and the mouse model, vaginal candiasis can be established by administering Candida cells to the vaginal cavity. Although these rodent systems have advantages, such as ease of use and access, low cost, and similar basic reproductive functioning to humans, both models come with serious limitations. Notably, vaginal Candida infections do not appear to occur naturally in either mice or rats. Infection can only be induced in an experimental setting, by regular treatment with the hormone estradiol to maintain a constant estrus state. A second difference, not to be neglected in infectious diseases research, concerns the microbial flora present in these animals. “The human vaginal microbiome is dominated by Lactobacillus species, setting it apart from most animal models tested so far,” states Gresnigt. The mouse and rat niche are populated by distinct bacterial species, such as Proteobacteria, Firmicutes, and Actinobacteria. “Considering the fact that changes in the host microbial flora play a significant role in establishing fungal infections, this difference should not be neglected.” A third important difference between rodent models and people concerns the immune system. In humans, the establishment of an infection is followed by activation of a local immune response. Immune cells rapidly infiltrate the vaginal tissue trying to clear the infection. “Surprisingly, this immune response is not effective,” says Gresnigt. “On the contrary, the discomfort and burning sensation women experience during an episode are not directly caused by the infection itself, but rather by the immune response.” Most of the current knowledge on the role of the immune response during vaginal Candida infections is based on experimental work with mouse and rat models. In mice, the recruited inflammatory cells appear incapable of deploying their antimicrobial mechanisms efficiently, whereas patient studies, although limited in number, suggest that the recruited immune cells do deploy their antimicrobial mechanisms, but at the wrong moment. The subtle, yet significant, differences in immunological communication networks between rodents and humans underscore the need to develop a humanized model capable of simulating and studying the immune response during vaginal candidiasis.
Moving towards organ-on-chip models
Organ-on-chip models have recently emerged as systems that can model more complex biological interactions in vitro. Therefore, these models are often considered as a valuable ethical alternative to experimental animals. They consist of miniature tissues that functionally and/or structurally represent human organs, cultivated on microfluidic chips. In addition to reducing the number of animals needed in research, the chips offer unique advantages that cannot be achieved in animal models. They allow for detailed examination of infections through microscopy and provide the ability to study isolated aspects of certain conditions, as parameters can be adapted precisely and readily. Recently, Gresnigt, together with an interdisciplinary group of researchers, used an intestine-on-chip model to microscopically characterize how antifungal drug administration via the bloodstream impacts Candida cells during an infection. Another advantage chip models offer, is the ability to add microbiota as well as probiotic bacteria through the microfluidics system. Thirdly, organ-on-chip systems open the possibility to transition towards more personalized medicine. Variability in the human population, in terms of genetic and physiological make up, can be mimicked. Despite their high potential, organ-on-chip models also have certain limitations. Due to the high complexity, researchers have not yet succeeded in incorporating all factors encompassing the innate and adaptive immune systems at the same time. Furthermore, with the rapidly evolving bioengeneering and microfluidics technologies, such endevors require highly interdisciplinary research collaborations.
Advancing Candida infection research
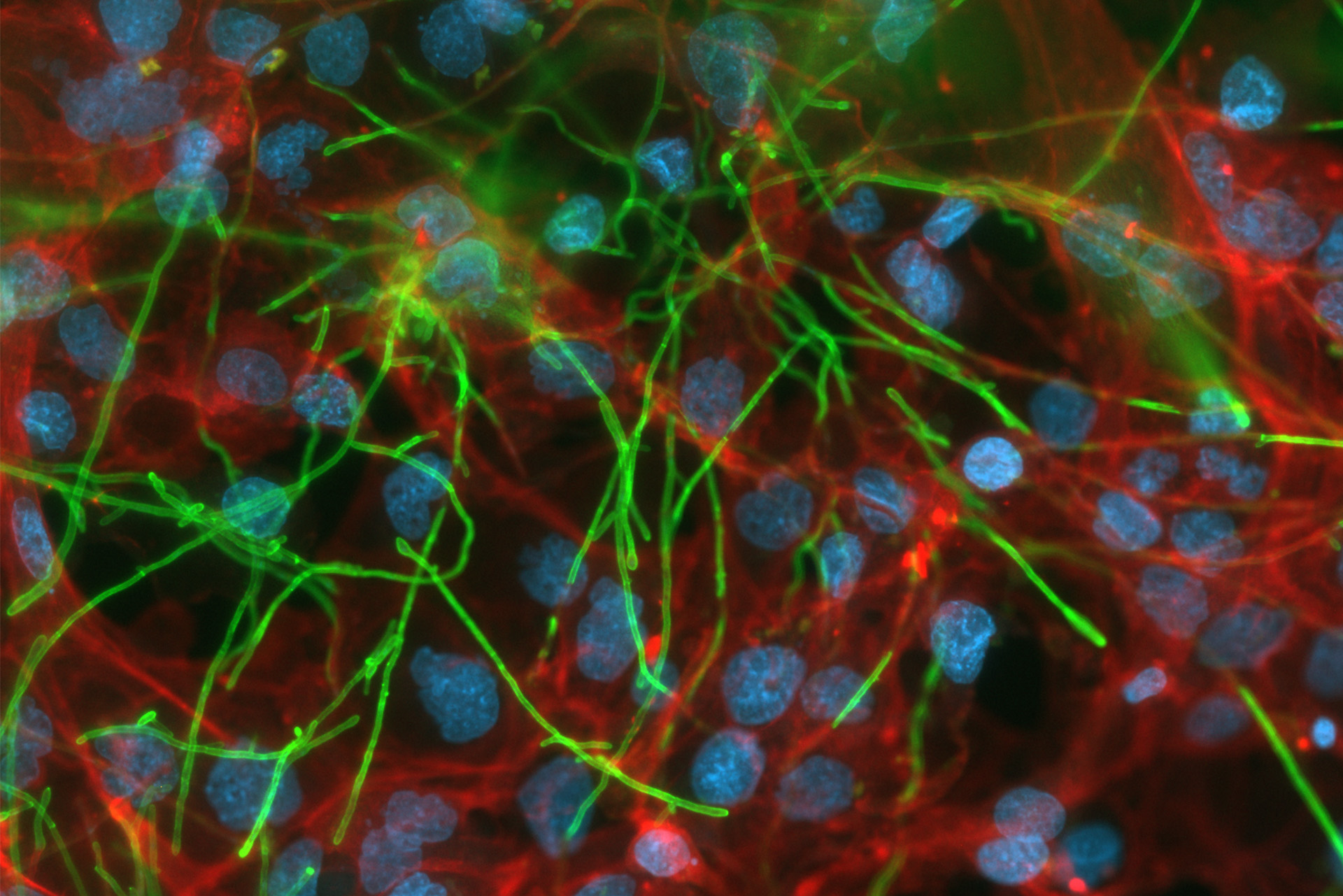
At the end of 2022, the first vagina-on-chip system was established and used in a pilot setup to study the interaction between the host and naturally-occuring bacteria. The model system included vaginal epithelial cells and stromal fibroblasts covering both sides of a porous membrane, and media representing the local nutrient availability. Selected microbial consortia were added, consisting of Lactobacillus crispatus and Gardnerella vaginalis. Although this system beautifully demonstrates how colonization with different bacteria shapes epithelial responses, a hallmark of vaginal yeast infections is the strong recruitment of neutrophils and their dysfunction in clearing the infection. The development of a humanized model to comprehensively investigate this has not been achieved to date, yet Gesnigt and his team offer hope. “We are establishing a model consisting of epithelial, endothelial, as well as immune cells of human origin, and will use this to study how human neutrophils respond to the vaginal Candida infection,” he says.

Vagina-on-chip model system. © Anna Schroll
The newly set up DeVEnIR project – an abbreviation of ‘Defining Vulvovaginal Candidiasis – Elements of Infection and Treatment’ – aims to optimize the vaginal candidiasis-on-chip model. The collaborative consortium consisting of KU Leuven, the University of Antwerp, the University Hospital of Antwerp, and Leibniz-HKI Jena, recently obtained Strategic Basic Research (SBO) funding from the Research Foundation – Flanders (FWO) to investigate the molecular and cellular basis of vaginal candidiasis, and explore new potential treatment methods. In a first phase of the project, vaginal samples will be collected from healthy women and patients in order to identify potential causative agents and/or biomarkers to aid diagnosis. In the subsequent phases, interactions between interesting microbial, chemical, and host factors will be investigated in the vaginal candidiasis-on-chip model. To stay updated on project progress or sign up for participation, subscribe to the newsletter.
“We are establishing a model consisting of epithelial, endothelial, as well as immune cells of human origin, and will use this to study how human neutrophils respond to the vaginal Candida infection.” – Mark Gresnigt, Leibniz-HKI